WELCOME
We are very pleased to welcome you all to the AHC & ATP1A3 10-year anniversary conference and 10th Symposium on ATP1A3 in disease. This year is the 10th anniversary of the discovery of the involvement of ATP1A3 in AHC. We are therefore especially happy to bring the community together again to review progress since gene discovery, and to think ahead to what the future might hold. We have an informative and wide programme considering developments from many aspects and we hope that you will find the meeting rewarding and enjoyable.
The Researchers and Clinicians, Organising Committee
The patient organisation representatives warmly welcome you to this important 10-year event. Whether in person in Edinburgh or online, we hope you will find this an informative and helpful event. Our aim for this 10-year anniversary conference and 10th Symposium on ATP1A3 in disease is to unite patients, families, researchers, clinicians, and all professionals involved in the lives of those living with AHC and ATP1A3 diseases. We have spent the past 12 months listening to what families and those with lived experience would like to gain from this event. It has been important for us to focus the start of each day on those living with the conditions or supporting them. We are 10 years on and still without a treatment specifically for these diseases. A wealth of research has been undertaken on meagre funds by dedicated researchers and clinicians. We want to unite the community to pause, reflect and join together to look at how to drive forwards research and clinical care for those with AHC and ATP1A3 diseases for the next decade. We are very aware that scientific conferences can be difficult for families and those living with the condition to attend. We hope that by making it accessible through an online option and translation we will be able to reach more of the international community. It is so important that we move forwards collectively and inclusively to tackle these devastating diseases.
Finally, we reflect on those with AHC/ATP1A3 diseases who should be with their families and are not. In 10 years, we have lost so many of our international community. We remember them in this event and hope that the research and clinical practice discussed over the three days will continue to inspire a renewed drive to improve the lives of those affected by and those living with these conditions.
The Patient Group Representatives’, Organising Committee
CONFERENCE AIMS
2022 marks 10 years since the discovery of the ATP1A3 gene. This was a scientific breakthrough in understanding the underlying aetiology of Alternating Hemiplegia of Childhood. This discovery involved collaboration on an international scale between researchers, clinicians and families living with the condition.
Over the last ten years, more has been discovered about Alternating Hemiplegia of Childhood and an expansion in the knowledge of further conditions now under the umbrella of ATP1A3 diseases. However, there is still no effective treatment for any of the ATP1A3 diseases including Alternating Hemiplegia of Childhood. There are many questions unanswered, and research is now highlighting avenues to explore as it is now recognised to be a multi-factorial condition with other systems, aside from the neurological system, being involved.
Discoveries such as the ATP1A3 gene being expressed in the heart have highlighted the cardiac risks involved in patients with this condition. There are other areas that still need more research including the respiratory and gastrointestinal aspects as well as being able to understand why sleep is critical in the recovery of some symptoms.
During the last ten years, the diagnostic criteria for AHC have been redefined and a number of new ATP1A3 diseases have been recognised. There is overlap in many of the ATP1A3 diseases and it is important to unite the international research, clinician, and patient communities around common goals.
The objective of this conference is to celebrate the significant achievements in research for AHC and ATP1A3 diseases but also allow an opportunity again for researchers, clinicians, and patient families to gather and consider the priorities for research (both from the experts’ and families’ perspectives) for the next ten years.
This event will help us identify the common goals for the next decade in AHC and ATP1A3 disease research. This is a unique opportunity to unite researchers, clinicians, and families around a common goal of developing research networks and research priorities for these rare neurological diseases (AHC and ATP1A3 diseases).
THE ATP1A3 IN DISEASE SYMPOSIUM:
How did it start and why it is important for AHC
Hendrik Rosewich, Karin Lykke-Hartmann, Kevin C. Ess and Tsveta Schyns-Liharska
The Standing Committee for the ATP1A3 in Disease Symposium
www.atp1a3-disease-symposium.org
We are delighted and grateful to see the 10th meeting of the Symposium taking place as part of the 10-year anniversary event in Edinburgh 19-21 October 2022.
In early 2012 several research teams in Europe, the USA and in Japan were racing to find the genetic causes of AHC. Thanks to the favourable research landscape, shaped during the preceding years by the joined efforts of AHC family organisations, clinicians and geneticists, each team was equipped with a collection of clinically characterised AHC blood samples that allowed them to apply the newly available WES technology and the trio sequencing approach. Soon, the news of breakthrough results by the USA-led team started to spread. Two publications, in Nature Genetics (Heinzen et al.) and in Lancet Neurology (Rosewich et al.), came out online on July 29th 2012, followed later by the publication on the Japanese patients (Ishii et al.). In all studies, heterozygous pathogenic mutations in the ATP1A3 gene were identified in the majority of the tested AHC patients. These publications could finally prove the uniform genetic basis of AHC. Importantly, heterozygous mutations in the ATP1A3 gene had already been described eight years earlier as the cause of an adult-onset movement disorder, rapid-onset dystonia-parkinsonism, RDP (Carvalho Aguiar et al. 2004).
Now, the path to designing new effective medical treatments tailored to AHC seemed open. It was evident that if we wanted to understand how ATP1A3 mutations can cause neurological disease, we have to better understand the role of ATP1A3 (and generally, of Na+/K+-ATPase) at the molecular and cellular level. We needed therefore to engage new relevant scientific and clinical expertise in the ongoing AHC research, and we knew that the best way to do that was through scientific meetings. In June 2012 ENRAH took the initiative and, together with the Duke University group led by David Goldstein, decided to organise a Symposium. There was no time to look for public funding, but we had to keep up the momentum of the breakthrough research going.
Moreover, we were very excited that some excellent research on ATP1A3 / Na+/K+-ATPase has already been produced over the years by various disciplines in this field. We started reaching out and inviting people the day after the first publications appeared online at the end of July 2012. The response that came was absolutely amazing, there was a high interest expressed by everyone contacted and new topics and research teams were added by the day. On August 20th we were ready to announce the Symposium in Brussels on 10 and 11 December 2012. The agenda, included 1) the recent findings of de novo mutations in ATP1A3 causing AHC2) clinical phenotypes studies in AHC 3) mutations in ATP1A3 causing RDP 4) functional studies of ATP1A3 mutations causing RDP 5) animal and cell models for ATP1A3 pathology. We will skip here the details on the challenges for the organisation the following three months, but it is important to acknowledge the financial support received for the Symposium from the AHC family organisations, that was critical to realize this meeting.
Sixty six participants from fifteen countries attended the Symposium in Brussels December 2012. These included scientists, clinicians and parents from AHC associations, and guests from the European Commission DG Research. What happened in Brussels is probably best described by one of our guests, who wrote us: “I have enjoyed to see this close cooperation between patient representatives and the scientific world. I have never experienced this kind of cooperation in this extent before.”
At the closing of the Symposium, we knew that this was rather a beginning, something we have to keep going and growing. Today, it is really amazing to see this long list of meetings and novel physicians, basic scientists, patients and family organizations joining at every meeting:
- Symposium ATP1A3 in Disease: From gene mutations to new treatments, Basil & Co Brussels Louise Seminar, Brussels, Belgium, 10 – 11 December 2012.
- Second Symposium on ATP1A3 in Disease: Genotype/Phenotype Correlations, Modelling and identification of potential targets for treatment, Catholic University School of Medicine, Rome, Italy 23 -24 September 2013.
- Third Symposium ATP1A3 in Disease: Genotype/phenotype correlations, modelling and identification of potential targets for treatment, de Lunterse Boer &Conf. Center De Werelt, Lunteren, The Netherlands, 29-31 August 2014.
- 4th Symposium on ATP1A3 in Disease: A Collaborative Effort of Advocates, Researchers and Clinicians to Set the Stage for Treatment Trials, Double Tree Bethesda Hotel Conference Center, Washington DC, USA, 27-29 August 2015.
- 5th Symposium on ATP1A3 in Disease, UCL Institute of Neurology, London WC1N 3BG, United Kingdom, 24-26 August 2016.
- 6th Symposium on ATP1A3 in Disease, Palace Hotel Tachikawa, Tokyo, Japan, 21-22 September 2017.
- 7th Symposium ATP1A3 in Disease, Robert H. Lurie Medical Research Center, Chicago, USA, 13-14 October 2018.
- 8th Annual Symposium on ATP1A3 in Disease: Moving towards the light, Grand Hotel Reykjavík, Iceland, 3-4 October 2019.
- 9th Symposium on ATP1A3 in Disease (ON-LINE), Karolinska Institutet & University Hospital, Stockholm, Sweden, 23-24 September 2021.
Each of these meetings has contributed in its unique way to the Symposium and we are grateful to all organisers and participants. We think this sets the basis for a community, that tries to join forces for our common goal: End ATP1A3-related diseases!
WELCOME TO EDINBURGH
Welcome to Edinburgh (both in person and online) for the AHC and ATP1A3 10-year anniversary conference and 10th Symposium on ATP1A3 in disease.
Edinburgh was voted the best city in the world in 2022 by Time Out. We hope you will agree, and we extend a warm Scottish welcome to you wherever you join us in the world. We hope you have time to stay on a few days and sample the delights of Edinburgh and Scotland.
We enclose some information below that should be helpful for you.
Arrival
The train station is central and approximately 5-10 minutes walk from the conference centre and 15 minutes from the Yotel Hotel on Queen Street.
The bus station is located at St Andrews Square and a few minutes’ walk from the conference centre (Royal College of Physicians of Edinburgh, RCPE) and a short walk from the hotel (Yotel) on the same street at the conference centre.
If arriving by flight, there are three options to reach the city centre where the hotel (Yotel) and the conference centre (RCPE) are. These are the airport bus, taxi, or the tram. All leave from just outside the airport terminal. The airport bus and the tram both stop in Princes Street which is two streets away from the conference centre and the hotel Yotel. If going straight to the hotel (Yotel) then the first stop on Princes Street is easiest and if going straight to the conference centre, the stop at St Andrews Square is nearest. Both those stops are about 5 minutes’ walk from the venue/hotel.
Conference venue
The Royal College of Physicians of Edinburgh has an accessible entrance via a lift. There are numerous lifts throughout the building for accessibility.
The main conference will be held in the Queen Mother’s Conference Centre (the main conference centre) in the college. Registration will take place outside the entrance door to this conference centre. Staff will direct you to the registration desks. Coffee will be served in the foyer next to the conference centre. The posters will be on display in this area also and we encourage everyone to view these.
For families attending with their children, there will be activities available in Meeting room 1 and 2 and you will receive more information on this by email. All children must be accompanied and supervised by an adult (parent or carer) during the whole of the conference. There will be a video linked TV in Meeting room 1 and 2 for parents/carers who are with their children to still watch the presentations in the main conference centre. They can also still ask questions via the slido app.
There is a first aid room that can be used by anyone with AHC/ATP1A3 if they need a quiet darkened room.
There are disabled toilets, but no “changing places”.
Conference venue tour
At the end of day 1 (Wednesday 19th October) at 4.30pm, there will be an opportunity for all delegates to go on a tour of the royal college. Parts of the Royal College date back to 1700 and have a wealth of history. The modern-day hypodermic needle (needle and syringe) was first invented in the Royal College of Physicians of Edinburgh 170 years ago in its previous location in Edinburgh. The inventor, Dr Alexander Wood, used the hypodermic needle to treat a patient’s neuralgia with morphine!
The college has the second-largest medical history library in the world. We are delighted that the librarian will show you the libraries and have a selection of books on display for you. Some of the libraries are unfortunately not fully accessible due to the age of parts of the building.
During the three days, the librarian will display several interesting historical medical books on neurology and genetics in reference to our conference. These will be available for viewing.
Conference dinner Thursday 20th October
If you have booked the dinner, please arrive at the Royal College for 7pm for your Scottish welcome. The old part of the building will be open for this. The old front door has steps, so if you require an accessible entrance, please use the same door as you used for the conference centre in the daytime and staff will escort you through.
The drinks reception will be in the New Library from 7pm. You will be escorted through to the Great Hall for the dinner. After dinner, at 9.30pm, there will be coffee in the New Library and musical entertainment before returning to the Great Hall at 10.30pm for a Scottish Ceilidh. If you have not attended a Ceilidh before, we look forward to welcoming you to your first and all instructions will be given by the caller, so you don’t need to worry! All abilities are welcomed.
Activities in Edinburgh… and a little of its history
Edinburgh has a wealth of tourist attractions. Here is a link to some of the top attractions:
https://www.visitscotland.com/destinations-maps/edinburgh/see-do/
We will also have some discounted attractions in your delegate pack on arrival.
If you require accessible information then there is a helpful guide, Euan’s guide which gives reviews on places and how accessible they are: https://www.euansguide.com/
Central Edinburgh is made up of ‘new town’ and ‘old town’. The ‘new town’ was built in the 1700s. The famous Royal Mile is in the ‘old town’ and spans the distance from Edinburgh Castle to Holyrood Palace. Princes Street, George Street and Queen Street (where the conference venue is) are the three main streets in ‘new town.’ It is easy to walk around Edinburgh, although a bit hilly in places! Arthur’s Seat is the high hill you will see in the city centre. It is an extinct volcano and has fantastic views at sunrise/sunset. We hope you have time after the conference to explore the history and delights that Edinburgh has to offer.
Wifi
Edinburgh is a wifi accessible city. Many places, including transport (e.g., buses) have free wifi to a limited download per day.
The conference venue has complimentary wifi.
Healthcare in Edinburgh
We hope you will be well during the conference and your stay, but if needed we have listed some important information below which might be particularly helpful for those travelling from abroad.
Emergencies:
For all emergencies in Scotland dial 999 for an ambulance
The children’s and adult’s emergency hospitals (accident and emergency) are in separate hospitals but all in the same area at Little France (on the outskirts of Edinburgh city). The children’s hospital is Royal Hospital for Children and Young People, and the adults’ hospital is the Royal Infirmary of Edinburgh. Both have Accident and Emergency (A&E) that are open 24/7.
Neurology at these hospitals is aware of this conference taking place and that those with AHC/ATP1A3 diseases will be visiting.
Non-emergencies:
If you require assistance that is not an emergency, then you can contact NHS 24 either online (https://www.nhs24.scot/) or via their 24/7 telephone helpline 111.
CONFERENCE PROGRAMME
| Wednesday 19th October: The Past – AHC and APT1A3, the last 10 years | |
| 09:00-10:00 | Registration and coffee for delegates and speakers
Main Foyer in Conference Centre |
| 09:00-09:30 | Opportunity for families to gather in person and discuss priorities for next 3 days
Meeting room 1/2 |
| Session 1. Alternating Hemiplegia of Childhood (AHC) andATP1A3: an overview
Chair: Professor Sanjay Sisodiya Lecture theatre and online |
|
| 10:00 | Welcome by organising committee. A reminder of why we are all gathering – focus on those with the lived experience of Alternating Hemiplegia of Childhood & ATP1A3 diseases
Katherine Behl, AHC UK and Conference organising committee |
| 10:10 | Keynote presentation:
ATP1A3 disease –phenotypic description to gene discovery Plenary talk discussing the basic science perspective of the gene discovery Professor Kathleen Sweadner, Harvard University |
| 10:40 | The evolving clinical spectrum of AHC and related conditions
Professor Hendrik Rosewich, University Medical Center, Goettingen |
| 11:00 | What is the role of ATP1A3?
Professor Poul Nissen, Aarhus University |
| 11:20 | Panel discussion |
| 11:30-11:45 | Coffee break, poster viewing and networking
Main Foyer in Conference Centre |
| Session 2: The development of animal models in the study ofATP1A3 diseases – what can they tell us?
Chair: Professor Arn Van den Maagdenberg Lecture theatre and online |
|
| 11:45 | State of the art historical overview on animal (mouse) models of ATP1A3-related disorders
Dr Steve Clapcote, University of Leeds |
| 12:15 | Panel discussion |
| 12:30 | The ATP1A3 Standing Committee
Dr Hendrik Rosewich and Dr Tsveta Schyns-Liharska |
| 12:45-13:45 | Lunch, poster viewing and networking
Main Foyer, Conference Centre |
| Session 3: Collaborative science – the AHC and ATP1A3 community and what it has brought
Chair: Johanna Brown Lecture theatre and online |
|
| 13:45 | Lived experience CAPOS
Ms Sonal Sumaria |
| 14:05 | The diagnostic criteria of AHC and ATP1A3 diseases
Professor Mohamed Mikati, Duke University, (Virtually) |
| 14:25 | What does it mean to have a ‘broken’ ATP1A3 pump?
Professor Arn Van den Maagdenberg, Leiden University Medical Centre |
| 14:45 | Panel discussion |
| 14:55-15:15 | Coffee break, poster viewing and networking |
| Session 4: Moving forwards towards new nosology and classification
Chair: Katherine Behl Lecture theatre and online |
|
| 15:15 | Day in the life of a parent……predictably unpredictable
Johanna Brown, AHC UK and Conference Organising Committee |
| 15:30 | Debate: What’s in a name? How should AHC be named and classified for families, clinical practice, and research?
Professor Sanjay Sisodiya Professor Hendrik Rosewich |
| 16:20-16:30 | Learning points from the day and Close |
16:30 Optional Tour of the Library at the Royal College of Physicians, Edinburgh
| Thursday 20th October: AHC & ATP1A3 diseases – where are we now, and where are we going? | |
| 08:00-08:30 | Registration and coffee for delegates and speakers
Main Foyer in Conference Centre |
| 08:25 | Opening of Day 2 – Lived experience of AHC and ATP1A3 diseases
Lecture theatre and online |
| Session 1: Sharing current research on AHC and ATP1A3 diseases: the life-course clinical perspective
Chair: Dr Simona Balestrini Lecture theatre and online |
|
| 08:30 | Why are natural history studies crucial for understanding the disease and potential future treatments? Learning from other rare conditions
Professor Andreas Brunklaus, University of Glasgow |
| 08:50 | Addressing the genotype-phenotype correlation in AHC and ATP1A3 diseases
Dr Aikaterini Vezyroglou, Great Ormond Street Hospital, University College London |
| 09:10 | ATP1A3 mutations cause polymicrogyria
Professor Renzo Guerrini, University of Florence, (Virtually) |
| 09:30 | Transition from childhood to adulthood
Dr Eleni Panagiotakaki, University Hospitals of Lyon |
| 09:50 | AHC – a lifelong disease. Long-term follow-up of adults with AHC
Dr Marco Perulli, Catholic University of The Sacred Heart, Rome |
| 10:10 | Panel discussion |
| 10:20-10:35 | Coffee break, poster viewing and networking
Main Foyer in Conference Centre |
| Session 2: Key dilemmas for clinicans, researchers, and families
Chair: Dr Aikaterini Vezyroglou Lecture theatre and online |
|
| 10:35 | How do we prevent delay in a diagnosis of AHC and ATP1A3 diseases?
Dr Ailsa McLellan, Royal Hospital for Children & Young People, Edinburgh |
| 10:55 | Sleep issues in AHC and ATP1A3 diseases
Dr Simona Balestrini, University College London and University of Florence |
| 11:15 | Treatment complexities in AHC and ATP1A3 diseases: dystonia management
Professor Manju Kurian, Great Ormond Street Hospital, University College London (Virtually) |
| 11:35 | Treatment complexities in AHC and ATP1A3 diseases: Flunarizine – to use or not to use?
Professor Masayuki Sasaki, Tottori University, Japan (Virtually) |
| 11:55 | How can we create a clinical trial for AHC and ATP1A3 diseases? Learning from other rare diseases
Professor Stéphane Auvin, Université de Paris |
| 12:15 | Panel discussion |
| 12:25-13:35 | Lunch, poster viewing and networking
Main Foyer, Conference Centre |
| Session 3: Back to the lab
Chair: Dr Steve Clapcote Lecture theatre and online |
|
| 13:35 | Rescue of Na2+/K+-ATPase mutational effects by secondary mutation: Perspective for future pharmaceutical intervention in ATP1A3 neurological disease
Professor Bente Vilsen, Aarhus University |
| 13:55 | Molecular mechanisms behind symptoms in ATP1A3 and 1 mutations
Professor Anita Aperia, Karolinska Institutet |
| 14:15 | ATP1A3 mutations cause dysfunction of motor networks within the spinal cord
Professor Gareth Miles, University of St Andrews |
| 14:35 | Updates from the TREAT AHC research study: what drugs are being tried?
Dr Danilo Tiziano, Catholic University of the Sacred Heart, Milan |
| 14:55 | Possible future therapeutic target? The γ-Benzylidene Digoxin Derivative BD-15
Dr Leandro Barbosa, Universidade Federal de São João del-Rei (Virtually) |
| 15:15 | Panel discussion |
| 15:15-15:30 | Coffee break, poster viewing and networking |
| Session 4: AHC and ATP1A3 diseases: many facets, many needs
Chair: Professor Helen Cross Lecture theatre and online |
|
| 15:30 | Introduction: The value of the Multi-Disciplinary Team (MDT)
Professor Helen Cross, Great Ormond Street Hospital, University College London |
| 15:40 | Cardiology, Professor Juan Kaski, Great Ormond Street Hospital, University College London |
| 15:50 | Gastroenterology, Professor Mohamed Mikati, Duke University, (Virtually) |
| 16:00 | Speech and Language therapy, Mr Steven Rose, Great Ormond Street Hospital, London |
| 16:10 | Physiotherapy, Dr Agnieszka Stępień, Józef Pilsudski University of Physical Education in Warsaw, Poland |
| 16:20 | Community Paediatrics/holistic palliative care, Dr Helen Aspey, Great North Children’s Hospital, Newcastle |
| 16:30 | Pain Medicine, Dr Suellen Walker, Great Ormond Street Hospital, University College London |
| 16:40 | Respiratory, Dr Don Urquhart, Royal Hospital for Children & Young People, Edinburgh |
| 16:50 | Psychiatry, Dr Boris Chaumette, Reference Center for Rare Psychiatric Diseases Paris |
| 17:00 | Panel discussion: Standard of care of AHC patients and development of clinical consensus for AHC/ATP1A3 diseases |
| 17:20-17:30 | Learning points from the day and Close |
Conference Dinner
19:00: Scottish Welcome Drinks reception, New Library, Royal College of Physicians, Edinburgh
19:20: Formal three-course dinner in the Grand Hall, Royal College of Physicians of Edinburgh
21:30: Coffee and drinks in the New Library
22:30: Scottish CeilidhPage Break
| Friday 21st October:
The Future for AHC/ATP1A3 diseases, clinical practice, and research |
||
| 08:00-08:30 | Registration and coffee for delegates and speakers
Main Foyer in Conference Centre |
|
| 08:30 | Opening of Day 2 – Lived experience of AHC and ATP1A3diseases
Memorial for those with AHC or ATP1A3 Diseases who have died Lecture theatre and online |
|
| Session 1: Driving forward research and understanding in rare diseases: how can patients and families be involved?
Chair: Katherine Behl Lecture theatre and online |
||
| 09:00 | Good Diagnosis: Improving the experience of diagnosis for people with rare conditions
Ms Natalie Frankish, Genetic Alliance UK |
|
| 09:20 | Patient-driven registries
Ms Isabella Brambilla, epiCARE patient rep and Dravet Syndrome registry co-ordinator |
|
| 09:40 | How to engage patients for faster transfer of research results to clinical practice
Claire Nolan, Head of Engagement, International Bureau of Epilepsy |
|
| 10:00 | Panel discussion | |
| 10:10-10:30 | Coffee break, poster viewing and networking
Main Foyer in Conference Centre |
|
| Session 2: Moving forwards: clinical trials
Chair: Dr Ailsa McLellan Lecture theatre and online |
||
| 10:30 | A clinical scale for AHC/ATP1A3 clinical trials
Dr Elisa de Grandis, University of Genoa |
|
| 10:50 | CBD in context in the management of rare epilepsies.
Professor Finbar O’Callaghan, Great Ormond Street Hospital, University College London |
|
| 11:10 | Panel discussion | |
| Session 3: Moving forwards: gene therapy strategies
Chair: Professor Arn Van Den Maagdenberg Lecture theatre and online |
||
| 11:20 | Learning from other neurological diseases – progress in gene therapy
Professor Mimoun Azzouz, University of Sheffield |
|
| 11:40 | AAV9-mediated ATP1A3 gene therapy: an update
Professor Cat Lutz, Jackson Laboratory (Virtually) |
|
| 12:00 | ATP1A3 gene editing: Using CRISPR for ATP1A3 diseases
Mr Alexander Sousa, Harvard University |
|
| 12:20 | Antisense oligonucleotide therapy: a possible target for AHC/ATP1A3 diseases
Professor Al George, Northwestern University |
|
| 12:40 | Panel discussion | |
| 12:50 | Prize for best poster | |
| 12:55 | Closure of conference, summary and key highlights of the conference and consensus on targets for future research
Summary by researcher, clinician, and patient organisation representative |
|
| 13:10 | Lunch
Main Foyer, Conference Centre |
|
SPEAKERS
Professor Anita Aperia
I am a senior professor in Pediatrics at the Karolinska Institute. Throughout my career, I have shared my time between clinical pediatrics and basic research, where focus has been on Na,K-ATPase. My maybe most important finding has been the demonstration that Na,K-ATPase is also a signal transducer that allosterically interacts with the IP3 receptor to regulate a number of vital functions. These days a large fraction of my time is also spent interacting with my four grandchildren, ranging in age between 1-21 years.
How dysfunction of mutated Na,K-ATPase alpha3 subunit can explain some major symptoms in alternating hemiplegia in childhood.
In healthy epithelial cells intracellular sodium (Nai) is constantly around 10mM. In neurons Nai oscillates between 10 and 40mM, a phenomenon that provides the driving force for neuronal activity. Nai values between 20 and 30 mM occur regularly at high neuronal activity. Those large variations in Nai require two Na,K-ATPase alpha subunits, alpha1 that is expressed in all cells and handles increases in Nai below 20mM and Na,K-ATPase alpha3 , that is only expressed in neurons, and that can return Nai ranging between 20 and 40 mmol to a resting value of 10mM.
High neuron activity is followed by a period of afterhyperpolarization, which is a major determinant of the firing rate of the neuron. Afterhyperpolarization is to a large extent determined by the activity of Na,K-ATPase, but the relative role of the different alpha subunits is unknown.
We have studied effects of alpha3 mutations on regulation of Nai in cultured neurons and the electrophysiology of motor neurons in mice carrying an alpha3 mutation. We found that when Nai was increased by brief exposure to a potassium free solution in neurons expressing mutant alpha3, the time to normalize Nai is significantly longer than in neurons expressing wild type alpha3. We have, in collaboration with Gareth Miles group, also carried out electrophysiological studies on motor neurons in wild type mice and in mice carrying a common RDP alpha3 mutation. Here we could demonstrate an almost complete absence of the afterhyperpolarization process in the motor neurons of mutant mice.
Diseases due to mutations in the protein PTTX are associated with similar but milder symptoms than AHC (dyskinesia and mild epilepsy) and have an onset in early childhood. In a recent study (Fabiano Benfenati and colleagues, Cell Death and Disease 2021) PTTX was shown to bind the Na,K-ATPase alpha3 protein and control its mobility in the cell. Silencing of PTTX was found to be associated with a 50% Na,K-ATPase alpha3-dependent loss of the afterhyperpolarization process.
Conclusion: The afterhyperpolarization process has a major influence on neuron activity and is critically dependent on intact Na,K-ATPase alpha3 function. Many symptoms in diseases due to alpha 3 mutation can be explained by loss of afterhyperpolarization. Since afterhyperpolarization is also determined by calcium activated voltage gated channels, more information about interaction between Na,K-ATPase alpha3 and these ion channels might open new pathways for more efficient symptomatic treatment of alternating hemiplegia in childhood.
Dr Helen Aspey
Helen was born in Durham and did not venture too far to study at Newcastle University Medical School. Helen developed an interest in paediatric palliative care whilst training as a community paediatrician under the guidance of her much-missed supervisor Dr Alison Guadagno. Helen was very proud to win the PAFTA ST4-8 award for her work within paediatric palliative care. In 2020 Helen was appointed Head of Department to the newly formed regional Children’s Holistic Integrated Palliative Care Service (CHIPS) whilst also working as a consultant community paediatrician based at the Great North Children’s Hospital. Helen feels very privileged to work within such a supportive and dedicated team and can’t wait to see how CHIPS will develop in the future. Helen’s other passion is Musical Theatre, performing in both professional and amateur productions across the North East, some favourites include Evita, Sister Act and West Side Story!!!
Role of the community paediatrician in management of AHC
- What is a community paediatrician?
- Explanation of my experience of being involved in the management of children with AHC.
- What role do they play in management of AHC? This can include symptom management, liaison with school providers, coordination of care, referral to other appropriate services, Development of Emergency Health Care plans ad transition to adult services.
- Discussion of the overlap between community services and paediatric palliative care. How may a paediatric palliative care approach benefit families.
Professor Stéphane Auvin
Stéphane Auvin, MD, PhD, FAES. Epileptologist and Child Neurologist. Full professor at Robert Debré University Hospital & Université Paris Cité, Paris, France. I am conducting the Epilepsy program and the center for rare epilepsies at Robert Debré University Hospital, member of the Epicare ERN. I have been appointed senior member of Institut Universitaire de France (2021- ). I am also conducting experimental research works in the INSERM U1141, Paris.
My clinical and research activities are focused on pediatric epilepsy, in particular infantile onset, and its treatments. My research team is working on antiepileptic drug development in the developing brain. The Epilepsy program at Robert Debré Children Hospital, Paris, is involved in antiepileptic drugs development and clinical trials (PK, Phase II, Phase III and Phase IV). I am author of more than 200 peer-reviewed papers or book chapters. I am glad to serve the ILAE (International League Against Epilepsy) as the chair of the regulatory affairs task force and the past-chair of the Pediatric commission (2017-2021) and as Deputy Editor for Epilepsia (2022-). I am member of the board of the ILAE French Chapter (2015- ) and the past-president (president 2019-2022) of the French Pediatric Neurology Society.
How can we create a clinical trial for AHC and ATP1A3 diseases by learning from other rare diseases
Clinical trials are used to study the effects of a therapeutic strategy. Their purpose is to explore or demonstrate the efficacy and/or safety of a treatment. There are different methods and designs, each with its own advantages and disadvantages. The highest standard for the demonstration of efficacy of a compound is usually a prospective randomized controlled trial design but this is not always doable. In the field of rare diseases, there are a few examples that have allowed the exploration of a molecule up to a clinical use. There are often particularities for clinical trials in rare diseases, the first of which is the low prevalence of the disease. The development of targeted therapies is a new hope for the emergence of new treatments through innovative designs with small numbers and also with comparisons to natural history cohorts. Based on past trials, we will review what might be proposed to explore treatment for AHC.
Page Break
Professor Mimoun Azzouz
Professor Azzouz obtained a Master’s in Neuroscience with 1st Class Honours from the University of Marseille in 1994. In 1997 he was awarded a PhD in Neuropharmacology at the University Louis Pasteur in Strasbourg. He then worked as a post-doctoral scientist at the Gene Therapy Center in Lausanne, Switzerland from 1997 to 2000. He was recruited in 2000 by Oxford oMedica plc as Senior Scientist and then appointed as Director of Neurobiology in 2003. In 2006, he was invited to join the University of Sheffield and was appointed as the Chair of Translational Neuroscience. His pioneering work, which has already produced major breakthroughs in animal models, has short and medium-term potential for real translation into major therapeutic advances for human neurodegenerative disease. Azzouz leadership has been recognized by several prestigious; e.g. ERC Advanced Investigator (2011) and ERC Proof-of-Concept (2017), IMI ARDAT (www.ardat.org) involving 34 partners and pharma companies to accelerate research & innovation of advanced therapies for rare diseases; JPND Award to develop better model systems for therapy testing; and the LifeArc/MRC Award and significant philanthropic gifts to establish a Gene Therapy Innovation & Manufacturing Centre (GTIMC) in Sheffield. GTIMC includes the provision of a state-of-the-art GMP manufacturing facility for gene therapy clinical vectors. He has been a key academic partner in the successful fundraising of £18M necessary to build the new Sheffield Institute for Translational Neuroscience (aN). Azzouz established significant esteem markers evidenced by membership for Panels/Boards of funding bodies, Scientific Advisory Board memberships, keynote and plenary lectures at established international meetings/institutions.
Translating Neurodegeneration: New Horizon for Gene-Based Therapeutics
This talk will give an overview and discuss the use of viral vectors for gene therapy in animal models of human diseases in particular neurodegeneration. In addition, using viral vectors, experimental models of disease including human cells and mouse model we will present and discuss mechanistic pathways and therapy development. Steps to translate gene therapy approaches into human clinical trials will be highlighted.
Dr Simona Balestrini
Simona Balestrini is Associate Professor of Child Neurology and Psychiatry at the Neuroscience Department, Children’s Hospital A. Meyer, and University of Florence. She is also Consultant Neurologist at the Chalfont Centre for Epilepsy and National Hospital for Neurology and Neurosurgery, UCLH, and Senior Clinical Research Fellow at UCL Queen Square Institute of Neurology. She has a special interest in epilepsy genetics, rare and complex neurodevelopmental disorders, and neurophysiology. She runs epilepsy clinics and epilepsy genomics clinics in Italy and in the UK. Her current research focuses on genotype-phenotype correlation studies, including the application of transcranial magnetic stimulation (TMS) to understand the underlying system abnormalities caused by genetic mutations, with the ultimate aim to translate the findings directly into personalised treatment.
Sleep issues in AHC and ATP1A3-diseases
One of the cardinal diagnostic criteria of AHC is the remission of hemiplegia and other paroxysmal events, but not seizures, with sleep, and their potential reappearance shortly after waking. Sleep induction is indeed used to end hemiplegic events.
There is evidence of altered sleep-wake patterns in children with AHC, with polysomnography recordings revealing the presence of frequent apnoea and arousals in a cohort of 22 children. Sleep disorders in AHC may be associated to behavioural and cognitive impairment. The complex interplay between hemiplegic events, sleep, epilepsy, cognition and underlying pathophysiology due to the underlying ATP1A3 mutation needs to be elucidated. There is some preliminary evidence of sleep disturbances in other ATP1A3-phenotypes and in other channelopathies distinct from ATP1A3-disease.
We recently conducted a retrospective study where we used clinical phenotyping, video-EEG data, and spectral analysis of sleep-, wake-, and ictal-EEG in a small cohort of AHC adult patients. We confirmed disrupted sleep persisting into adulthood, with sleep interrupted by frequent arousals. We also demonstrated a neurophysiological lateralisation of ictal power preceding hemiplegic episodes, detectable with scalp EEG despite no electrographic epileptiform activity. Based on these preliminary results, we are conducting further studies to characterise the clinical and EEG phenotype of sleep in AHC and other ATP1A3, which may further elucidate pathophysiological mechanisms and inform treatment strategises.
Professor Leandro Barbosa
Dr. Leandro Barbosa has a PhamaD degree and a Ph.D. in Biochemistry and has expertise in the study of biochemistry and physiology evaluation of the involvement of the Na,K-ATPase in health and disease, such as cancer and hematological disorders. During his career, he has published 70 manuscripts in peer-reviewed journals and written 2 book chapters, with an H index of 15 and more than 400 citations. Some important awards that he was awardee: The international recognition he received by winning the 2016 New Investigator Award of The American Physiological Society – Cell and Molecular Physiology Section – of which he was the first scientist working outside the United States to be awarded, and the research productivity fellowship award which he received from the CNPq (Brazilian equivalent to the NIH), that has the objective of recognizing and valuing the work of highly productive scientists invested in advancing scientific knowledge and technological innovation.
BD-15: a digoxin derivative that increases the a3-Na,K-ATPase activity and has a neuroprotective effect. Is it a possible treatment for AHC?
Digoxin and other cardiotonic steroids (CTS) exert their effect by inhibiting Na,K-ATPase (NKA) activity. CTS bind to the various NKA isoforms expressed in different cell types, giving CTS its narrow therapeutic index. We have synthesized a series of digoxin derivatives (g-Benzylidene digoxin derivatives) with substitutions in the lactone ring (including non-oxygen and ether groups), to obtain CTS with better NKA isoform specificity. Some of these derivatives show some NKA isoform selective effects. One of particular interest is BD-15, which demonstrated in SF9 cells expressed the a1-3 isoforms, BD-15 was able to increase specific a3 activity of NKA. A molecular docking approach favored NKA isoform-specific interactions for the compounds that supported their observed activity. Moreover, BD-15 was tested in Wistar rats for 3 days in IP treatment in 20, 100, and 200 μg/Kg concentrations and BD-15 did not alter the behavior of rats treated with different doses. An increase in the specific α2,3-Na, K-ATPase activity was again observed for all doses of BD-15 tested in the hippocampus and prefrontal cortex. Subsequently, when the effect of BD-15 on cardiac tissue was analyzed we did not find any signal of cardiotoxicity and cell death. The cell cytotoxicity was tested on cancer cell lines and BD-15 exhibited low cytotoxicity in tumor and non-tumor cells, presenting IC50 values of 8 μM, while digoxin showed cytotoxicity at nanomolar concentrations. Another important effect of BD-15 is the neuroprotection effect found in the chemical ischemia model in N2a cells and in a global ischemia model in Wistar rats, where BD-15 significantly prevented cell death caused by ischemia. BD-15 can be a promising drug for AHC treatment since it increases the activity of the α3-Na,K-ATPase in the hippocampus and prefrontal cortex has a neuroprotection effect, and decreases oxidative stress in these brain regions.
Mrs Katherine Behl
Katherine Behl is mum to a child with AHC who is 5 years old. Following diagnosis she was keen to engage with the patient organisation groups. She is a trustee of Alternating Hemiplegia of Childhood UK Charity where she is Vice-chair and Research representative. She recently became President of AHC Federation of Europe (AHCFE). She is keen to raise awareness of AHC and ATP1A3 diseases and has created some information leaflets and videos for families and professionals, some in collaboration with other rare disease organisations and also EpiCARE. She sits on the All Parliamentary Cross Party working group on rare diseases in Scotland which has recently fed into the revised UK Rare Diseases Framework.
In 2021, the film about her family experiences of AHC reached the final of the Berlin Rare diseases film festival.
Professionally, she is a physician in Internal Medicine and Geriatric Medicine currently taking a secondment to undertake clinical research and a PhD in dementia.
Event Opening
This presentation will open the three-day event. It will discuss the aims and the input from the different groups (researchers, clinicians, and patient groups) has helped shape this event. It will discuss the importance of focusing on those living with the conditions and their families in progressing understanding and developments in AHC and ATP1A3 diseases. A short video of the Human Timebombs will follow.
Ms Isabella Brambilla
Isabella Brambilla is the mother of three children, whose youngest is affected by Dravet Syndrome, a rare form of drug-resistant epilepsy. She founded Dravet Italia Onlus1 in 2010 to support research and to improve the patient’s quality of life. With a Scientific Committee establishing the “National Register of Dravet Syndrome and other Syndromes related to mutation of the gene SCN1A and PCDH19”2 now being developed at European level2. She also promoted other surveys at international level (Falls with Epilepsy, Vaccination, Emergency Protocol, Covid19 all for DS). She is working to update Dravet Diary App (iOS, Android and web) easy-to-use family tool to manage DS pa-tient’s activities, appointments, medical examinations, and seizures. In 2013, she has contributed to the co-foundation the Dravet Syndrome European Federation of which she was first deputy and then chair until 20203.She organized “Horizons for Dravet Syndrome”4 meetings on Dravet’s Syndrome and has contributed to several scientific publications. She is a Coordinator ePAG in ERN EpiCARE5 and member of Steering Committee sins 2017. In February 2019, she become part of the new group “ePAG-Italia”6. In 2020 she has contributed to the co-foundation the European KCNQ2 Association7In 2021 she has contributed to foundation the Rare and Complex Epilepsies Alliance8.
Patient-driven registries
Clinical registers and patient databases are key tools for the development of clinical research in the field of rare diseases, to improve patient care and healthcare planning.
They are the best way to pool data to obtain a sufficient sample size for epidemiological and/or clinical research.
Registries serve as a recruitment tool for launching studies focusing on disease aetiology, pathogenesis, diagnosis or therapy.
The collection of clinical data, therapies, longitudinal evolution, observational studies and/or data collection for phenotype definition and research developments.
The data collected through natural history studies will allow us to fully define the spectrum and clinical course of Dravet syndrome. Furthermore, the registers will be valuable for future clinical studies, including potential genetic therapies, and for post-authorisation safety studies (PASS).
The Platform-Residras Registry will be an important tool for systematising data on Dravet Syndrome and related syndromes in order to improve understanding of the disease and knowledge of related research.
The reports and updates of the collected data, which are indispensable for promoting knowledge of the disease and its social and economic impact, will be made available to all interested parties, with the hope of promoting and supporting scientific research and with the aim of discovering innovative therapeutic options and their evolution over time for the management of DS and related syndromes.
I believe that this Registry is also crucial to monitor the early recognition of the various and complex associated comorbidities.
All this information and related studies will have the ultimate goal of giving patients the best possible quality of life behind epilepsy.
New treatment options certainly offer concrete new benefits in controlling epileptic seizures, but not for all patients. The pathology registry www.dravet-registry.com with regular annual follow-ups will allow to add evidence of efficacy in patients and to analyse the most effective therapeutic combinations. It will also monitor long-term safety and efficacy as well as any other behavioural and psycho-intellectual benefits.
Despite the difficulties given by the GDPR and the approval times of the ethics committees of individual hospitals and the common consensus of specialists and patient associations, it is essential to create a strong network that works as a team to speed up as much as possible this collection of data, which in my opinion is the only way to optimise knowledge, stimulate research and monitor the efficacy of new drugs and learn about side effects, as well as an analysis of the evolution of the pathology in the long term. This will lay the foundation for future and more precise studies and developments of new precision and future gene therapies.
We patients strongly want to give you all the information we can to help us give our children a better future.
Dr Jo Brown
Jo Brown is the Treasurer for AHC UK and part of the Organising Committee for this conference. Her goddaughter has a diagnosis of AHC, and this led to her becoming involved with AHC UK. She is a Consultant Forensic Psychiatrist based in Edinburgh where she is Clinical Director and lead consultant for the Forensic Women’s Service. As a qualified Cognitive Analytic Therapist, she works with military veterans at Veterans First Point. She is involved in teaching at Edinburgh University and has an Honorary Fellowship with the School of Law. She is the Chair of the Forensic Faculty of the Royal College of Psychiatrists in Scotland and has participated in responding to several recent Scottish Government reviews including the Scott Review of Mental Health Law and the Barron Review of Forensic Mental Health Services in Scotland.
Day in the life of a parent: Predicably unpredictable
When you are part of the team putting an event like this together, it becomes an opportunity to add in some of the perspectives of the parents and families who are part of the patient organisations.
This session has arisen through discussions within the patient organisations sharing their experiences as they have worked together to bring this event to fruition. This sharing of experiences has alerted us to the variation in symptoms, systems of care and support. It has also highlighted the shared experiences that parents, and primary caregivers have.
We agreed that it was important to share this at the conference – to highlight what it is to be a parent of a child with AHC or an ATP1A3 disease, navigating systems and advocating for the lives of children and adults. These are the personal views of parents contributed anonymously for the purpose of this event. We hope that it will provide a wider context in which you, as clinicians and researchers, can work with the families and individuals experiencing these conditions.
Professor Andreas Brunklaus
Professor Andreas Brunklaus, is a consultant paediatric neurologist at the Royal Hospital for Children, Glasgow and honorary professor at the College of Medical, Veterinary and Life Sciences, University of Glasgow. He trained at the Charité Medical School, Humboldt University Berlin, Germany and completed his child neurology training at Great Ormond Street Hospital in London and the Royal Hospital for Children in Glasgow. He obtained his MD from the University of Glasgow and has an ongoing research interest in epilepsy genetics, in particular sodium channel disorders. He is an international expert in SCN1A-related epilepsies and Dravet syndrome and is the author of several key publications. He leads international research collaborations developing cutting edge diagnostic tools in epilepsy genetics and is the UK chief investigator for “SCN1A Horizons – A natural history study of SCN1A-related epilepsies in the United Kingdom”. He is lead of the Scottish Paediatric Epilepsy Network, has numerous national and international advisory board positions, is co-chair of the International League Against Epilepsy (ILAE) Task Force on Clinical Genetic Testing and editorial board member of the European Journal of Paediatric Neurology.
Why are natural history studies crucial for understanding the disease and potential future treatments? Learning from other rare conditions
The National Institutes of Health (NIH) defines a natural history study as a pre-planned observational study intended to track the course of the disease. Its purpose is to identify demographic, genetic, environmental, and other variables (e.g., treatment modalities, concomitant medications) that correlate with the disease’s development and outcomes. Natural history studies are likely to include patients receiving the current standard of care and/or emergent care, which may alter some manifestations of the disease.
Mutations in the gene encoding the α1 subunit of the voltage gated sodium channel (SCN1A) are associated with several epilepsy syndromes. These range from the severe infantile onset epilepsy, Dravet Syndrome to relatively mild phenotypes found in families with genetic epilepsy with febrile seizures plus (GEFS+). Research suggests that the epilepsy as well as the many comorbidities are related to the underlying sodium channel dysfunction. Despite evidence-based treatments, the outlook for individuals with Dravet syndrome remains extremely poor and there is a need for more effective treatment of SCN1A-related epilepsy not only addressing the seizures but also addressing the cognitive, behaviour and motor difficulties. In order to be able to evaluate the efficacy of any treatments in SCN1A/DS robust natural history data are required measuring both seizure burden as well as cognition and associated comorbidities.
The speaker will introduce the concept of natural history studies and give examples of recent natural history studies and which lessons can be drawn from previous research in the field.
Dr Boris Chaumette
Boris Chaumette, laureate of the Ecole de l’INSERM, obtained a MD with a specialty in psychiatry and a PhD in neurobiology in 2016. After a post-doc at McGill University (Montreal-Canada) and a competitive position in France (CCA INSERM Bettencourt), he has been recruited as associate professor at the Université Paris Cité and the GHU Paris Psychiatrie & Neurosciences (Ste Anne Hospital). Since 2019, he leads a Center for Rare Psychiatric Disorders at Paris (France). This Center performs clinical genetic assessments and provides therapeutic advice for the management of patients with rare genetic diseases and psychiatric/behavioural symptoms.
His research at the Institute of Psychiatry and Neuroscience of Paris (INSERM U1266) aims at discovering new genetic and epigenetic factors for psychiatric disorders. He has co-authored more than 40 scientific publications. Among his work, he reported that ATP1A3 is a gene involved in certain rare forms of psychosis, unusually starting during childhood. This contributed to extend the phenotype of this condition to non-motor symptoms.
The need for an MDT to manage AHC – how should this be composed? Psychiatry
Whereas motor symptoms are reported for a long time in ATP1A3 mutation carriers, the description of behavioural and psychiatric symptoms is more recent. Previously, when exploring rare and uncommon forms of schizophrenia starting during childhood, we identified genetic variants in ATP1A3 and its gene interactors. These mutations could associated with purely psychiatric presentation without motor symptoms. This contributed to extend the phenotype of ATP1A3 mutations.
Since 2019, thanks to a partnership with the French Association for Alterning Hemiplegia, we are following patients carrying ATP1A3 mutations at our Center for Rare Psychiatric Disorders (Ste Anne hospital, Paris, France) and are reporting the behavioural and psychiatric features. While the psychotic symptoms (hallucinations, delusion) seem to be quite rare, other symptoms request psychiatric care: autism spectrum disorder, attentional deficit with hyperactivity disorder in children, depressive and anxiety symptoms in adolescence or young adulthood. But the most impactful manifestions are the behavioural outbursts.
Sadly, there is no current specific treatment for behavioural and psychiatric symptoms for ATP1A3 patients and the care has to rely on trials and errors strategies. Based on our clinical experience and through a multidisciplinary approach, we will try to propose the use of some pharmaceutical drugs. Besides, psychosocial interventions could be very helpful for patients and caregivers.
Acknowledging psychiatric disorders in ATP1A3 should pave the way for early and individualized treatments, in concertation with caregivers. Systematic psychiatric screening should be proposed for each patient with ATP1A3 mutation.
Dr Steve Clapcote
Dr Steve Clapcote was born in Poole and studied at the University of Liverpool before undertaking research fellowships at the University of Oxford followed by Mount Sinai Hospital, Toronto, where he began working on ATP1A3. He has been a lecturer in pharmacology at the University of Leeds since 2008.
State of the art historical overview on animal (mouse) models of ATP1A3-related disorders
The molecular mechanisms through which mutations in ATP1A3 result in a broad range of neurological symptoms are poorly understood. However, in vivo comparative studies using genetically altered model organisms can provide insight into the biological consequences of the disease-causing mutations in the neurone-specific α3 subunit of the Na+,K+-ATPase pump. This talk will review the existing mouse, zebrafish, Drosophila and Caenorhabditis elegans models used to study ATP1A3-related disorders and discuss their potential contribution towards the understanding of disease mechanisms and development of novel therapeutics.
Professor Helen Cross
Professor Helen Cross is The Prince of Wales’s Chair of Childhood Epilepsy, and Director at UCL-Great Ormond Street Institute of Child Health, as well as Honorary Consultant in Paediatric Neurology Great Ormond Street Hospital for Children NHS Foundation Trust, London and Young Epilepsy, Lingfield, UK. Her research has been targeted at improving outcomes in early onset epilepsy, specifically in assessing the role of surgery and ketogenic diet. She has also established an interest in ATP1A3 associated disorders. She has held key leadership roles both nationally and internationally. She is currently President of the International League Against Epilepsy 2021-2025. She developed, as Coordinator, the European Reference Network for Rare and Complex Epilepsies (EpiCARE) launched in 2017.
The need for an MDT to manage AHC – how should this be composed?
What makes a good MDT? Discussion from clinicians involved in MDT management of AHC and ATP1A3 diseases on how different specialties can feed into the MDT at a local and national level.
Dr Elisa De Grandis, MD, PhD
Elisa De Grandis is a Child Neuropsychiatrist Consultant at the Division of Child Neuropsychiatry, Gaslini Children’s Hospital, and Associate Professor at the University of Genoa, Italy. After the degree in Medicine and Surgery in 2001, she developed a deep interest in the field of Movement Disorders and rare diseases. She is author of n. 45 indexed scientific publications, and she is involved in many International Research Groups. She is Italian Node Coordinator for the INTERNATIONAL CONSORTIUM FOR THE RESEARCH ON ALTERNATING HEMIPLEGIA OF CHILDHOOD AND OTHER ATP1A3-RELATED DISEASES, Data Manager for the Italian Biobank and Clinical Registry for AHC and Scientific Coordinator for the Italian AHC Association (AISEA).
A clinical scale for AHC/ATP1A3 Clinical Trials
Over the last few years, several rare disorders have found promising or effective treatments that have led to a substantial improvement in the quality of life of patients and their families. A critical node in a rare disorder such as Alternating Hemiplegia of Childhood (AHC), in the view of forthcoming candidate treatments, is the lack of disease-specific clinical outcome measures. Patients are currently evaluated by functional scales that are suitable for the evaluation of a single type of movement disorder or have been conceived for other conditions. Moreover, most of these rating scales were specifically designed for adult patients.
On the clinical side, great efforts have been done over the last 10 years by the clinical groups working on AHC to reconstruct the natural history of the disease, through the setup of international registries of patients and longitudinal retrospective natural history study. Nowadays, we know that AHC patients display a very complex phenotype including different paroxysmal disturbances, epileptic seizures, tonic, and dystonic attacks, plegic episodes, dysautonomic features, abnormal ocular movements, and migraine. Both chronic movement disorders and paroxysmal attacks evolve in frequency and type in relation to age.
Given these premises, in the setting of a granted project focused on the pre-clinical identification of candidate treatments (TREAT-AHC) and to address the requirements of regulatory agencies for registration purposes, an AHC specific scale has been developed.
The AHC scale, specifically meant for clinicians, has been designed by 4 experts in AHC and revised by other 2 experts in movement disorders. The scale addresses all the disease domains and can be divided into 4 parts: PAROXYSMAL SCORE, EPILEPSY SCORE, NON-PAROXYSMAL SCORE, ADAPTIVE SCORE.
Besides of being of key relevance in the evaluation of the efficacy of candidate compounds during forthcoming clinical trials, the AHC scale will allow clinicians worldwide to evaluate the rate of disease severity, the semeiology of the disease, the genotype-phenotype correlation, the natural evolution of the AHC during the lifespan of a patient (for example: the improvement of the activities of the daily living, of the neurological chronic symptoms…).
The scale will be tested and validated on Italian, French and Spanish AHC patients. All patients will be examined and filmed according to a standardized video protocol. The different Patient Advocacy Organizations (Italian (AISEA), Spanish (AESHA) and French (AFHA)) have been actively involved in the design, management, and logistic support for the project.
Ms Natalie Frankish
Natalie Frankish is the Policy and Engagement Manager for Scotland for Genetic Alliance UK, the largest alliance of organisations supporting people with genetic, rare and undiagnosed conditions in the UK. Genetic Alliance UK advocates for fast and accurate diagnosis, good quality care and access to the best treatments. The organisation actively supports progress in research and engages with decision makers and the public about the challenges faced by our community. Genetic Alliance UK runs two long standing projects:
Rare Disease UK: A campaign focused on making sure the new UK Rare Diseases Framework is as successful as possible, and to ensure that people and families living with rare conditions have access to a final diagnosis, coordinated care and specialist care and treatment.
SWAN UK: The only dedicated support network in the UK for families affected by a syndrome without a name – a genetic condition so rare it often remains undiagnosed.
Good Diagnosis: Improving the experience of diagnosis for people with rare conditions
The UK Rare Diseases Framework recognises the value of early diagnosis of rare conditions and identifies getting a faster diagnosis as a priority. But speed of diagnosis is only part of the picture. How a person is supported on their journey through diagnosis is equally important and all too often people with rare conditions report feeling unsatisfied with their experience of diagnosis. Genetic Alliance UK set out to better understand people’s experience of diagnosis and to identify what matters most to people on their diagnosis journey.
In November 2021, Genetic Alliance UK issued a call for people with lived experience of rare conditions to participate in the Good Diagnosis project. A total of 43 people agreed to participate and were invited to attend one of three online workshops. Each workshop invited participants to reflect on their experiences at three key stages of the diagnosis journey: the search for a diagnosis, receiving a diagnosis, and following a diagnosis. Each workshop was recorded, and the discussions reviewed to identify key themes.
The dominant themes that emerged from our workshops were:
- A person’s experience of diagnosis was significantly influenced by the healthcare professionals involved in their care.
- Clinicians who are aware and informed of rare conditions would likely be able to identify and accept the possibility of a rare condition quicker, and set in motion the referrals or tests needed to make a diagnosis faster.
- When healthcare professionals have access to reliable information and sources of support, they are better equipped to inform and support people living with rare conditions.
The Good Diagnosis project confirmed that there is an urgent need to equip healthcare professionals to better support people with rare conditions.
Professor Al George
Dr. George is the A.N. Richards Professor and Chair of the Department of Pharmacology, and Director of the Center for Pharmacogenomics at the Northwestern University Feinberg School of Medicine. He has been a pioneer in elucidating the genetics and pathogenesis of channelopathies with a focus on neurodevelopmental disorders. His research on AHC focuses on understanding fundamental molecular and cellular mechanisms, and on testing new therapeutic strategies by using human neuron models of the disease.
Antisense oligonucleotide therapy: a possible target for AHC/ATP1A3 diseases
Antisense oligonucleotides (ASO) have emerged as effective therapeutic tools for genetic disorders of the nervous system. One strategy utilizing this technology seeks to reduce messenger RNA (mRNA) levels of a heterozygous ‘toxic’ gain-of-function variant to prevent expression of the disease-causing allele. Conceptually, this same approach may work for a dominant-negative variant if the wild-type (WT) allele alone can encode sufficient levels of the protein for normal physiology. Pathogenic variants in ATP1A3 are the major cause of AHC, and there are genetic and in vitro experimental observations that suggest a dominant-negative mechanism. Whether suppression of the mutant allele would restore normal ATP1A3 function in neurons is not known. This talk will present an approach to evaluating the effects of ASO molecules on AHC patient-specific induced pluripotent stem cell (iPSC) derived neurons. The principle goal of this work is to determine if selective targeting of a mutant ATP1A3 allele is feasible, and if knocking down this allele affects protein levels and function. This proof-of-concept study may help determine if an ASO strategy is worth pursuing for AHC.
Lay abstract
This presentation will explain the basis for a treatment strategy that uses antisense oligonucleotides or ASO. This strategy has been approved for treating genetic disorders of the nervous system, and it has become an attractive approach for developing treatments for other brain diseases. An ASO is a short, synthetic molecule combining pieces of RNA and DNA. ASO are used to target a gene’s messenger RNA (mRNA), which cells use as a blueprint for making a specific protein. Targeting mRNA with an ASO can cause it to be eliminated. For ATP1A3 disorders, the goal is to test if an ASO can selectively target the copy of the gene’s mRNA with the disease-causing mutation, but not affect the normal copy. If this is feasible, then the effect of eliminating the mutant mRNA on the biochemistry and function of ATP1A3 can be assessed. This study will help determine if an ASO strategy might work for AHC.
Professor Renzo Guerrini
Prof. Renzo Guerrini is Director of the Neuroscience Department at the Children’s Hospital A. Meyer, Florence, Italy. His previous Academic positions include Professorships at University of Pisa, King’s College London and University College London. His research focuses on the neurophysiology, neurogenetics and the treatment of pediatric epilepsies. He has coordinated the Commission of Pediatrics of the ILAE and has been the principal investigator of DESIRE (Development and Epilepsy – Strategies for Innovative Research to improve diagnosis, prevention and treatment in children with difficult to treat Epilepsy), a major EU Research project. He received the Ambassador for Epilepsy ILAE Award, 2003, the American Epilepsy Society’s Clinical Research Recognition Award, 2012 and the Elisa Frauenfelder Prize on Research and Innovation, 2019. He has co-authored over 500 papers in Peer-reviewed journals and 12 books. His Official H-Index is 110.
ATP1A3 mutations cause polymicrogyria
Professor Juan Kaski
Dr Kaskiis Associate Professor of Paediatric Inherited Cardiology at the UCL Institute of Cardiovascular Science, where he leads the UCL Centre for Paediatric Inherited and Rare Cardiovascular Diseases, and Consultant Paediatric Cardiologist at Great Ormond Street Hospital (GOSH), London, UK. He is the Director of the GOSH Centre for Inherited Cardiovascular Diseases. His clinical and research interests are focused on the clinical and genetic characterisation of inherited cardiovascular disease and sudden cardiac death in childhood. He is immediate past-Chair of the Association for European Paediatric Cardiology (AEPC) Working Group on Genetics, Basic Science and Myocardial Disease and sits on the Executive Board of the European Society of Cardiology (ESC) Cardiomyopathy and Myocarditis Registry Programme and the ESC Council on Cardiovascular Genomics. He leads an international paediatric HCM consortium of over 45 centres, which was responsible for the development of the first sudden death risk prediction model for childhood HCM. He is Chair of the Task Force for the 2023 European Society of Cardiology Cardiomyopathy Guidelines.
The need for an MDT to manage AHC – how should this be composed? Cardiology
Alternating hemiplegia of childhood (AHC) is a rare disorder caused by de novo variants in the ATP1A3 gene, expressed in neurons and cardiomyocytes. The disorder is characterized by early-onset, recurrent, often alternating, hemiplegic episodes; seizures and non-paroxysmal neurological features also occur. Dysautonomia may occur during hemiplegia or in isolation. Premature mortality can occur in this patient group and is not fully explained. Preventable cardiorespiratory arrest from underlying cardiac dysrhythmia may be a cause. We have previously shown that electrocardiographic (ECG) abnormalities are common in individuals with AHC, with characteristics similar to those of inherited cardiac channelopathies and that may reflect impaired repolarisation reserve. Furthermore, the risk of life-threatening cardiac rhythm abnormalities is equivalent to that in established cardiac channelopathies (≈3%). Sudden cardiac death due to conduction abnormality has also emerged as a seizure-related outcome in murine Atp1a3-related disease. The dynamic ECG and neurological features point to periodic systemic decompensation in ATP1A3-expressing organs, and raise the possibility that cardiac dysfunction may account for some of the unexplained premature mortality of AHC. Systematic cardiac investigation is warranted in alternating hemiplegia of childhood, as cardiac arrhythmic morbidity and mortality are potentially preventable. My talk will summarise the cardiac features of AHC and discuss the role of the cardiologist in the multidisciplinary team assessment of individuals with AHC.
Lay summary
Alternating hemiplegia of childhood (AHC) is a rare genetic condition that is associated with early onset of seizures, and can be associated with a risk of unexplained death. We have shown that patients with AHC commonly have abnormalities of the heart’s conduction system, that may be associated with a higher risk of abnormal heart rhythms. The features are similar to genetic heart diseases that are associated with an increased risk of abnormal heart rhythms, many of which can be treated. In this talk, I will summarise the cardiac features of AHC and discuss the role of the cardiologist in the assessment and management of patients with AHC.
Professor Manju Kurian
Professor Manju Kurian is a Professor of Neurogenetics and NIHR Research Professor at UCL-Great Ormond Street Institute of Child Health. She is also a Consultant Paediatric Neurologist at Great Ormond Street Hospital.
After graduating from Cambridge University, she trained in Paediatrics before subspecialising in Paediatric Neurology. At the end of her clinical training, she undertook a PhD (University of Birmingham) investigating the molecular genetic basis of childhood neurological disorders (2007-2011). She moved to UCL after her PhD and is now an independent Principal Investigator at the Institute of Child Health. She has been awarded a Wellcome Intermediate Fellowship (2012-2017), L’Oreal For Women in Science Award (2017), NIHR Professorship (2017-2022), ICNA Jon Stobo Award (2018) and the The Jules Thorn Award for Biomedical Research (2019-2024). Her grant income exceeds £10 million, and she has >170 peer reviewed publications including works in Nature Genetics, Science, Science Translational Medicine and Lancet Neurology.
Her current research encompasses gene discovery for childhood neurological disorders, including early onset epilepsy, neurodegeneration and movement disorders. Her lab uses mainly cell models to investigate the underlying pathological basis of disease. She works closely with UCL Gene Therapy groups to develop novel therapeutic strategies for children with pharmacoresistant movement disorders. Her long term goal is to translate her research for patient benefit, through improved clinical diagnosis and precision medicine approaches.
Treatment complexities in AHC and ATP1A3 diseases: dystonia management
Alternating Hemiplegia of Childhood (AHC) and other ATP1A3-related phenotypes represent a group of complex neurological conditions associated with a broad spectrum of movement disorders. Many affected children experience dystonia – repetitive involuntary twisting movements or postures as part of their clinical condition. In this talk, I will outline general management strategies as well as more specific measures used to symptomatically manage dystonia in this group of conditions.
Professor Cat Lutz
As a neuroscientist and geneticist by training, Cat Lutz has worked extensively with mouse models of neurodegenerative diseases, with a focused emphasis on SMA, Friedreich’s Ataxia and ALS. Her lab has worked to model in mice, many of the genetic forms of these diseases and has ensured that these preclinical mouse models are available globally to the scientific community to accelerate discovery and treatments. From 2015-2022 she established and was the Senior Director, of the In Vivo Pharmacology Efficacy Testing Service at JAX, where she designed preclinical platforms for use in testing therapeutics for industry and academic partners. Cat now serves as the Vice President for the Rare Disease Translational Center at JAX, where she continues to study and develop resources for the ALS and other rare neurological disorders.
Mouse models for AHC past, present and future directions
While the development of high-throughput sequencing technology and its application to clinical diagnostics has yielded the genetic basis for many rare genetic diseases, the development of effective treatments has not kept pace. As our understanding of the underlying causes of rare genetic diseases continues to expand, the potential for genome based therapy solutions is an attainable reality. However, much work remains to be done to surmount the considerable challenges in developing such therapies, and mouse models play a critical role in the understanding of disease pathophysiology and mechanism, in addition to serving as patient avatars for disease modifying therapeutics. The Jackson Laboratory Rare Disease Translational Center (RDTC) serves as a repository for existing mouse models, and a strategic partner for rare disease foundations for new model development and preclinical therapeutic testing. This talk will provide an overview of the current AHC models at the RDTC, current therapeutic strategies and new directions for models.
Dr Ailsa McLellan
Dr Ailsa McLellan is a Consultant Paediatric Neurologist at the Royal Hospital for Children and Young People in Edinburgh. She trained in Paediatrics in Edinburgh, Cardiff and Dundee and in Paediatric Neurology in Dundee, Edinburgh and Great Ormond Street Hospital. Dr McLellan’s area of particular expertise is in Paediatric Epilepsy and she manages a tertiary level epilepsy service with outreach to hospitals in the South-East of Scotland. She manages the teenage epilepsy service, epilepsy transition services, Vagus Nerve Stimulation service and the Ketogenic Diet service. She is lead clinician of the Scottish Paediatric Epilepsy Surgery Service. She is interested in education in epilepsy and participates widely in teaching events locally and nationally. She is involved in the development of Paediatric Epilepsy Training (PET) courses in the UK and internationally. She is Training Programme Director and Head of School for paediatric training in South-East Scotland.
Her research interests include epilepsy and behaviour, epilepsy and cognitive outcomes, epilepsy and sleep, aetiology of epilepsy, SUDEP and seizure detection.
She was a founder member of the Scottish Paediatric Epilepsy Network (SPEN), lead clinician of SPEN from 2007-2011 and continues to sit on the Advisory Group, Steering Committee and lead on different work streams. She is a member of the Scottish Intercollegiate Guidelines Network (SIGN) guideline development group for Epilepsies in Children and Young people. She is the past Chair of the British Paediatric Epilepsy Group (special interest group of the British Paediatric Neurology Association) and is currently Professional Support Officer for the BPNA and on the Executive Council of the BPNA. She is also a trustee of Epilepsy Scotland.
How do we prevent delay in a diagnosis of AHC?
The symptoms of AHC present in infancy, a time when paroxysmal events are very common affecting 9% of infants. The range of paroxysmal events in infancy is vast and the features of different paroxysmal events in infancy can look very similar. Misdiagnosis of paroxysmal events is common, including misdiagnosis of AHC.
Paroxysmal events typically present to primary care physicians and Emergency Department (ED) doctors initially. These professionals may then refer the infant on to a general paediatrician. The general paediatrician may then refer the infant on to a paediatric neurologist for assessment. This process typically takes time, and even when the infant is referred on to paediatric neurology the diagnosis may still not be made initially. The symptoms of AHC can be subtle and misinterpreted, and even the classical symptoms may initially be considered as a feature of an alternate diagnosis by experienced paediatric neurologists.
The history of the presenting features will always be a key part of the diagnosis of AHC. Home video recordings of typical events are also extremely useful from a diagnostic point of view. The use of secure video transfer systems to allow parents/carers to send in videos to their clinical teams has allowed more timely and accurate diagnoses of paroxysmal events, as well as the potential for peer review.
In order to prevent delay in diagnosis referral pathways are necessary so that infants with uncertain paroxysmal events access a medical professional who has knowledge of the disorder. There is increasing use of home videos of paroxysmal events and primary care physicians and ED doctors should encourage parents/carers to make videos of paroxysmal events in their infants so these are available to the medical professionals they refer them on to. Education is required for general paediatricians and paediatric neurologists to highlight the range of features of the condition, including the more subtle presentations so that there is a high index of suspicion. Timely diagnosis by an experienced clinician is important to avoid unnecessary investigations and treatment and to facilitate the appropriate management to be initiated for the infant and their family.
Professor Mohamed Mikati, MD
Mohamad Mikati MD is the Davison Distinguished Professor of Pediatrics, Professor of Neurobiology, Chief, Division of Pediatric Neurology at Duke University (since 2008). His basic research centers, in animal models, on mechanisms of, and neuroprotection from, seizure related neuronal injury in the developing brain and on animal models of Alternating Hemiplegia of Childhood (AHC) and its gene therapy. His clinical research has focused on characterization and therapy of pediatric epilepsy syndromes and on clinical manifestations therapy and natural history of AHC. He has more than 300 original articles (H index 61), trained in Neurology at Mass General, in Neurophysiology at Boston Children’s (BCH), was assistant then associate professor at Harvard, director of research in the Epilepsy Program at BCH, Chairman of Pediatrics at the American University of Beirut, his MD alma mater. He is the recipient of multiple national and international honors including the Harvard Community HP Peer Recognition, Debs, Hans Zellweger, Hamdan and Michael Frank research and achievement awards. Davison Distinguished Professor of Pediatrics, Professor of Neurobiology
Chief of Pediatric Neurology,
Director of the AHC Multidiscipinary Clinic and Program
Director of the Pediatric Epilepsy Program
Duke University
How gastroenterology Can be of Benefit to the Multidisciplinary Team- from a Neurologist’s Perspective
Gastroenterological problems are common and require attention and management in most patients with AHC. In our recent study (Orphanet J Rare Dis. 2020 Sep 3;15(1):231. doi: 10.1186/s13023-020-01474-w.) of 44 consecutive AHC patients we found that 41/44 (93%) exhibited gastrointestinal symptoms requiring medical attention. For these 41 patients, symptoms included constipation (66%), swallowing problems (63%), vomiting (63%), anorexia (46%), diarrhea (44%), nausea (37%), and abdominal pain (22%). Symptoms indicative of dysmotility occurred in 33 (80%). The most common diagnoses were oropharyngeal dysphagia (63%) and gastroesophageal reflux (63%). 16 (39%) required gastrostomy and two fundoplication. Severity of gastrointestinal symptoms correlated with non-paroxysmal neurological disability index, Gross Motor Function Classification System scores, and with the presence/absence of non-gastrointestinal autonomic dysfunction (p = 0.031, 0.043, Spearman correlations and 0.0166 Cramer’s V, respectively) but not with the paroxysmal disability index (p = 0.408). The Multidisciplinary approach to AHC care was developed at Duke directly after the gene discovery in 2012 in partnership of CureAHC and was published in 2017 (Masoud et al. Curr Treat Options Neurol (2017) 19:8doi: 10.1007/s11940-017-0444-7) and has been continuously refined. Currently we have as part of that team 3 gastroenterologists covering each an area of importance for AHC patients: motility, nutrition and liver/general gastroenterology as well as a dietitian who specializes in the ketogenic diet. Key to success is the close interaction between these specialists and the four-member coordinating/leadership team (AHC program director, co-director, triage nurse, scheduling assistant) AHC program, which also closely works with the family and with local providers. Research and publications are essential for keeping the members of the multidisciplinary team engaged and in advancing knowledge about GI and other specialty problems in AHC. This also applies to other subspecialties such as cardiology, sleep, developmental pediatrics, psychiatry, psychology, endocrinology, genetics, pulmonary, speech, physical and occupational therapy. Thus, our experience indicates that: GI problems are common in patients with AHC and should be part of the initial and subsequent evaluations follow up and management. Most of these problems can be managed with advice and medications but in a minority of may require targeted surgical procedures.
The Diagnostic Criteria of AHC and ATP1A3 Diseases
Diagnostic criteria are a set of signs, symptoms, and tests to define a disease in question and to be used for clinical practice. Diagnostic criteria need to be distinguished from classification criteria which are standardized definitions that are primarily intended to create well-defined, relatively homogenous cohorts for clinical research. Diagnostic criteria are meant to be broad with high sensitivity and high negative predictive value as to not exclude individuals with possible disease. Classification criteria are meant to have high specificity and high positive predictive value so as to include patients only with definite disease and, thus, may inadvertently exclude patients who actually have the disease for the sake of homogeneity for studies. In a disease with very high sensitivity and specificity, diagnostic and classification criteria converge and, thus in that case, would be the same and the same criteria would then be used in clinical practice and in clinical research. The Aicardi diagnostic criteria of AHC were used to ascertain patients for the gene discovery work as well as for subsequent studies and in clinical practice. There were subsequent restatements with some adaptations of these criteria in 2014 and 2019. However, as has happened for many other diagnostic criteria for other diseases such as the DSM and its revisions in psychiatry the expanding and evolving knowledge of the manifestations of AHC and of disorders that mimic AHC supports revising the existing criteria. This also applies to other ATP1A3 related diseases (RDP, CAPOS, RECA, FIPWE, D-DEM∅). Revising AHC criteria is also supported by the fact that the presence of the ATP1A3 mutations is proving to be helpful in AHC clinical diagnosis and by the fact that there is confusion in interpreting data in the literature in which patients who have had episodes of hemiplegia on either side and are children are considered to have AHC. The point is that not every alternating hemiplegia during childhood is Alternating Hemiplegia of Childhood. The first i.e. hemiplegia that alternates sides, is a symptom that can exist in multiple non AHC disorders such as Moya Moya disease and hemiplegic migraine while the the second, i.e. AHC, is a disorder. We, thus, have proposed a revision of the Aicardi criteria (Eur J Paediatr Neurol. 2021 May;32:A4-A5.) and hope that this starts a process that would lead to consensus about the diagnostic criteria for AHC and ATP1A3 related disease. Among the different methods to create consensus among experts that will be presented, a Delphi method process is likely to prove most suitable for that process.
Professor Gareth Miles
Professor Miles began his research career in New Zealand where he completed his PhD. He then undertook postdoctoral training at Dalhousie University, Halifax, Canada before beginning a lectureship at the University of St Andrews in 2007. He is currently Professor of Neuroscience and Head of the School of Psychology & Neuroscience at the University of St Andrews. Professor Miles’ research aims to decipher how networks of neurons within the spinal cord and brainstem control movement and how dysfunction in these neural networks contributes to a range of neurological disorders.
ATP1A3 mutations cause dysfunction of motor networks within the spinal cord
Neurological disorders associated with mutations in the ATP1A3 gene, which encodes for the α3 subunit isoform of the sodium-potassium pump, typically present with motor symptoms. We have recently shown that sodium-potassium pumps containing the α3 subunit (α3-NKA) play an important role in the regulation of spinal motor circuits via the production of a pump-mediated ultra-slow afterhyperpolarisation (usAHP). We therefore investigated whether dysfunction of neural networks within the spinal cord contributes to the motor symptoms of ATP1A3-related disorders. We addressed this by using a novel mouse model harbouring the T613M mutation of the ATP1A3 gene, which is found in patients with Rapid-Onset Dystonia-Parkinsonism. We show that animals with the T613M mutation are hyperactive and have gait disturbances. When examining neuronal properties and circuit function in isolated spinal cord preparations, we observed a complete lack of usAHPs in T613M-affected lumbar neurons, and a reduced capacity for spinal motor networks to regulate rhythmic motor output in response to large increases in intracellular sodium. We suggest that this deficiency is caused by a reduced capacity for mutant α3-NKA to extrude sodium and therefore maintain sodium homeostasis. Overall, our work provides critical new information supporting a role for spinal neurons in ATP1A3-related disorders and suggests that spinal circuits should be considered when developing new therapeutic strategies.
Lay summary
Although disorders associated with mutations in the ATP1A3 gene typically involve problems with movement, little consideration has been given to the potential contribution of the spinal cord to these disorders. We have therefore investigated whether neurons of the spinal cord are affected by mutations in the ATP1A3 gene. We have achieved this by using genetically modified mice harbouring an ATP1A3 gene mutation that is found in patients with Rapid-Onset Dystonia-Parkinsonism (RDP). We found that animals with this mutation are more active and have abnormal patterns of walking. When examining the properties of neurons within the spinal cords of these animals, we found that the neurons lacked certain properties that would normally enable them to adjust to the demands of rigorous activity. We therefore believe that dysfunction within the spinal cord may contribute to the motor symptoms seen in ATP1A3-related disorders and that the spinal cord represents an important additional target that should be considered when designing therapies.
Claire Nolan
Head of Engagement, International Bureau of Epilepsy
Claire joined the health charity sector in 2011 building a bowel cancer community awareness initiative, a research network of people affected by Parkinson’s and a Patient and Public Involvement (PPI) in research programme. Claire’s more recent work as a freelancer and working for a company MediPaCe saw her working to support pharmaceutical companies and people affected by health conditions to work together to better design and deliver industry initiatives as well as working with several UK and European patient organisations and the Charities Research Involvement Group (CRIG) in the UK on projects related to how charities and pharmaceutical companies can better collaborate.
She has recently joined IBE as Head of Engagement and her remit will include developing a Global Epilepsy Advocates programme to ensure people affected by epilepsy are equal stakeholders in research, policy and healthcare.
How to engage patients for faster transfer of research results to clinical practice
Professor Poul Nissen
Poul Nissen received his PhD in 1997 at Aarhus University for work on the ternary complex of translation elongation factor EF-Tu with GTP and aminoacylated tRNA, and did postdoctoral work 1997-2000 at Yale on the ribosome with Tom Steitz (Nobel Prize 2009). In 2001 he established his own lab at Aarhus University with a focus on P-type ATPases including Na,K-ATPase, for which his lab determined the first crystal structures in 2007. He was the director of the PUMPkin center of excellence of the Danish National Research Foundation 2007-2017, and is currently the director of the neuroscience center DANDRITE, which is the Danish node of the Nordic EMBL Partnership, and of the Danish national cryo-electron microscopy facility (EMBION).
Nissen’s research focus on Na,K-ATPase is on the human isoforms in brain, in particular ATP1A3 and how it is mechanistically affected by AHC mutations.
What is the role of ATP1A3?
Professor Finbar O’Callaghann
Finbar O’Callaghan is Professor of Paediatric Neuroscience at the UCL GOS Institute of Child Health, University College London and (Hon) Consultant Paediatric Neurologist at Great Ormond Street Hospital. He was President of the British Paediatric Neurology Association (BPNA) (2018-2020) and formerly Secretary of the European Paediatric Neurology Society. Finbar initially graduated from Oxford with a MA (Hons) in Modern History in 1983 before going on to study medicine at Bristol Medical School, graduating in 1990. He worked as a junior paediatrician in Bristol, Bath and Birmingham before undertaking a Fellowship in Neonatology at the Hospital for Sick Children in Toronto, Canada. On returning to the UK, he was awarded a Wellcome Trust training fellowship in clinical epidemiology, completing an MSc in Epidemiology at University of London and Diploma of London School of Hygiene and Tropical Medicine, before working with the MRC Environmental Epidemiology Unit in Southampton. This was followed by appointment as Senior Clinical Research Fellow at the National Perinatal Epidemiology Unit, University of Oxford where he ran the Oxford Registry of Early Childhood Impairment (ORECI). He was awarded his PhD in Clinical Epidemiology from the University of Bath in 2002. Finbar completed his paediatric neurology training in Southampton and was appointed Consultant Paediatric Neurologist in Bristol in 2003 before moving to London in 2013. Currently his clinical work is based predominantly at Great Ormond Street Hospital but he continues to run the supra-regional tuberous sclerosis clinic in Bath.
Finbar’s research has been in the area of neuroepidemiology and clinical trials. He has particular clinical and research interests in infantile epilepsy, tuberous sclerosis complex and stroke. He is currently developing trials to investigate the use of cannabis based medicinal products in refractory early-onset epilepsies and genetic generalized epilepsies.
CBD in context in the management of rare epilepsies
In the 21st Century there has been increased interest in the use of cannabis based medicinal products in the treatment of refractory epilepsies. The Cannabis plant produces in excess of 140 cannabinoids but almost all clinical research to date has focused on two: cannabidiol (CBD) and tetrahydrocannabinol (THC). In humans the vast majority of clinical research in the context of epilepsy has focussed on the use of CBD. Since 2017, there have been five published double blind, randomised controlled trials (RCTs) of the use of pure CBD (Epidyolex ®) as a treatment in three rare conditions characterised by refractory epilepsy: Dravet syndrome, Lennox Gastaut syndrome and Tuberous Sclerosis Complex. There have been several open-label non-randomised non-controlled studies of the same CBD product (Epidyolex ®) used in other clinical scenarios i.e. treatment resistant epilepsies in general, CDKL5 disorder, Doose syndrome, Aicardi syndrome, Duplication of 15q, tuberous sclerosis complex and FIRES syndrome.
This talk will examine the science behind the use of CBD as a treatment for epilepsy. It will attempt to analyse the existing evidence for the efficacy of CBD in the treatment of rare epilepsies and to place it in context vis a vis other treatment options.
Dr Eleni Panagiotakaki
Dr Eleni Panagiotakaki, senior Paediatric Neurologist – Epileptologist, is in charge, since 2008, of the
Paediatric Clinical Epileptology unit, at University Hospitals of Lyon (HCL). She received her MD degree from the University of Patras, Greece and her PhD in « Correlation of the genotype in Wilson’s disease with the clinical and biochemical phenotype » from the Athens Medical School, where she also completed her Paediatric Training. From 2004-2008, she worked as a Pediatric Neurologist at Robert Debré Hospital, in Paris. She was among the main partners of the ENRAH (European Network for Research on Alternating Hemiplegia, 2005-2011), and nEUroped projects (European Network of Rare Paediatric Neurological Diseases) financed respectively by the 6th and 7th Framework European programmes. She is one of the founding members of the International Consortium for Research on Alternating Hemiplegia of Childhood (www.iahcrc.net) created in 2014, and the leader of the phenotyping projects. She has recently (2022) been elected Deputy coordinator of the IAHRC. She is the Principal Investigator in Hospices Civils de Lyon in all studies concerning Alternating Hemiplegia.
Transition from childhood to adulthood
Since the description of Alternating Hemiplegia of Childhood (AHC) by Verret and Steele in 1971 and with the acceleration of the recognition and diagnosis of new patients after the identification of ATP1A3 gene in 2012, the number of patients having been recognized as having AHC is growing exponentially. A lot of medical professionals are discovering and encounter patients with the disorder and due to recent advances in medical care, even children with the most severe forms of the disorder grow into adulthood. The challenge now is to achieve the transition of their health management from paediatric to adult health services in the more appropriate and beneficial way.
Transition is defined as “the purposeful, planned movement of adolescents and young adults with chronic physical and medical conditions from child-centred to adult-oriented health-care systems” (Blum et al., 1993).
But this process is often done, in a non-organized way, that fails to meet the needs of this multidimensional disorder in adulthood and can be the source of a lot of stress for the patient and his family. Adult healthcare providers may not have sufficient knowledge about the disease and special centres with multi-disciplinary teams are harder to find for adult patients comparing to the care available for children.
Recommendations or Guidelines on how to perform transition for AHC patients is lacking, because of the extreme rarity of the disorder and the fact, that the health policies and available personnel and structures can widely differ between countries. On the other hand, several rare genetic neurological diseases share common features and care needs, and AHC patients can profit from existing experience and already published strategies.
In France existing practices of transition can be resumed to the following: 1) absence or delay of transition and continuation of the care by a Paediatric Neurologist that has a good knowledge of the disease. 2) transfer of the care to a General Practitioner. 3) transfer to an adult specialist physician, mostly a neurologist specialized either in epilepsy or in movement disorders. 4) transfer of the care, to the health practitioner that supervises a special residence ‘home’ for adults with a handicap.
All these solutions could be acceptable, depending on differences of health offer from one region to the other. Nevertheless, it is high time to establish standards for transition process, trying to meet the special needs and particularities of the disorder.
Dr Marco Perulli
I am a paediatric neurologist with a particular interest in genetics and neurophysiology. I completed my clinical training as paediatric neurologist and psychiatrist at Catholic University of Sacred Hearth in 2019 and I will soon discuss my PhD in neuroscience at the same institution. Between 2019 and 2020 I did a research fellowship at UCL where I discovered the fascinating world of AHC. I contributed to observations regarding the clinical course of the disease, and I am finalizing a study on the mechanism of disease explored in vivo with neurophysiology (TMS-EEG).
AHC – a lifelong disease. Long-term follow-up of adults with AHC
Background: Although described as non-progressive, alternating hemiplegia of childhood (AHC) can display sudden deterioration, anecdotally reported mainly in childhood. Outcome in adulthood is uncertain.
Objectives: To describe the long-term follow-up of neurological function in adults with AHC.
Methods: Seven adults with AHC were included in this retrospective single-centre study. Clinical history and previous investigation data were gathered from review of medical records. Video-documented neurological examination was performed at the last follow-up visit in 4/7.
Results: Over a median follow-up of 16 years, neurological outcome and trajectories were heterogenous. All individuals showed new neurological signs or symptoms. Three patients experienced a serious irreversible neurological deterioration after prolonged quadriplegic episodes and/or status epilepticus in their second or third decade. One individual died aged 29.
Conclusions: This video-series suggests that AHC in adulthood is not stationary; larger cohorts are needed to identify genotype-phenotype correlations and clinically useful outcome predictors.
Lay Summary
As suggested by its name, most of the knowledge gathered in alternating hemiplegia of childhood (AHC) is from childhood. Our aim was to describe the progress of the condition in adulthood with the goal of increasing awareness among adult neurologists, helping to recognise and possibly prevent clinical manifestations in adult age. We describe the clinical course of the condition in seven people with a very long follow up. We video-recorded people at the last follow-up to document variability of the neurological features. In the group we show that all the affected people developed new problems compared to what they experienced during childhood and some of them showed sudden or progressive deterioration of their abilities. This suggests that AHC can continue to change into adulthood, evolving with aging and with variable long-term outcomes. A better understanding of factors involved in the long-term progression of the condition will help to develop and monitor better treatments.
Mr Stephen Rose
Steve is a speech and language therapist with more than 20 years’ experience of working within the disability field and experience of working with children with rare conditions. He is the lead SLT for the epilepsy service at GOSH, he was previously Head of the Children’s Specialist Services at Sense and has a special interest in children and young people with deafblindness and multiple disabilities. He is a national advisor to the Royal College of Speech and Language Therapists for multiple disability/deafblindness.
The role of the Speech and Language Therapist (SLT) in the Multi-disciplinary Team
Considering the role of the SLT in management of support in the context of the how to manage this condition with variability in presentation and possible regression. Exploring how SLT’s can contribute within an MDT when likely to have had no previous experience of this condition.
Professor Hendrik Rosewich, MD
Hendrik Rosewich, MD is an associate Professor and assistant Medical Director, pediatric neurologist and senior physician in the Department of Pediatrics and Adolescent Medicine, Division of Pediatric Neurology at the University Medical Center Goettingen at Georg August University in Goettingen, Germany. From the very beginning, his clinical and basic research interests focused on the etiology and pathogenesis of rare neurometabolic diseases including peroxisomal disorders like Zellweger-Syndrome Spectrum and X-linked Adrenoleukodystrophy (X-ALD) as well as rare movement disorders like alternating hemiplegia of childhood (AHC). Besides primary care for affected children with these disorders, he is especially interested in the investigation of the pathogenesis of Alternating hemiplegia of childhood and X-linked Adrenoleukodystrophy to answer essential questions for the development of therapeutic options.
Another clinical research focus lies in the field of chronic inflammatory CNS diseases, especially paediatric Multiple Sclerosis and he serves as the Deputy Medical Director of the German Centre for Multiple Sclerosis in Childhood and Adolescence.
The evolving clinical spectrum of AHC and related conditions
Since the discovery of the ATP1A3 gene as the primary genetic defect of Alternating Hemiplegia of Childhood (AHC) in 2012, a variety of phenotypes with mutations in this gene have been described, making it difficult for paediatric and adult neurologists to diagnose a disease from the ATP1A3-related disease spectrum. This has enormous implications for patients who do not belong to one of the classical ATP1A3 related disease entities (AHC, rapid-onset dystonia-Parkinsonism (RDP), cerebellar ataxia, areflexia, pes cavus, optic atrophy, sensorineural hearing loss (CAPOS) and others), because these patients still have to wait a long time for a diagnosis with a variety of severe symptoms. Many of the phenotypes described as new are not new at all and can already be found in the first descriptions of the disease by Jean Aicardi more than 40 years ago. The systematic review of all described phenotypes with the aim to identify a core phenotype will be the major task of the ATP1A3 community in the next years. Based on this core phenotype, diagnostic criteria can then be defined that can identify a mutation in the ATP1A3 gene with a high probability. That this task will be a great challenge is shown by the findings of the last years. While the majority of phenotypes caused by mutations in the ATP1A3 gene show a combination of movement disorders, seizures, cognitive impairment and behavioural abnormalities, phenotypes with severe developmental impairment due to neuronal migration defects have been identified. The rapid development of this phenotypic variability also poses enormous challenges to basic science. Only the precise analysis of the dysfunctions of the encoded protein resulting from the mutations, including upstream and downstream effects, will allow the development of causal therapies. However, the knowledge gained in the last 10 years gives hope that this could be achieved in the near future.
Professor Masayuki Sasaki
Present Position Director, Department of Child Neurology, National Center of Neurology and Psychiatry, Kodaira, Tokyo, Japan
Education
1983 MD, Niigata University School of Medicine, Niigata, Japan
1983-1988 Residency of Pediatrics, Niigata University Hospital
1988-1990 Residency of Child Neurology, Department of Child Neurology, National Center of Neurology and Psychiatry, Kodaira, Tokyo, Japan
1990-1992 Fellow, Division of Inherited Metabolic Disorders, National Institute of Neuroscience, National Center of Neurology and Psychiatry
1992-1994 Visiting fellow, Myelin Section, Laboratory of Molecular and Cellular Neurosciences, National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Health (NIH), Bethesda, Maryland, USA
Academic Appointments
1994-1996 Full time doctor, Department of Child Neurology, National Center of Neurology and Psychiatry
1996-2002 Section Chief, Department of Child Neurology, National Center of Neurology and Psychiatry
2002-Present Director, Department of Child Neurology, National Center of Neurology and Psychiatry
Treatment complexities in AHC and ATP1A3 diseases: Flunarizine – to use or not to use? – From our experience on flunarizine in patients with AHC in Japan –
Since flunarizine’s effectiveness on alternating hemiplegia of childhood (AHC) was reported by Casaer and Azou1), 23 patients with AHC started using flunarizine in Japan. Sakuragawa reported that flunarizine effectively decreased the frequency and severity of hemiplegic attacks in three-fourths of the patients2). However, in 1999, the pharmaceutical company withdrew the production and sales of flunarizine due to its side effects. Suddenly we could not get flunarizine in Japan.
This withdrawal caused most patients using flunarizine to stop. After discontinuing flunarizine, some patients presented with increased frequency and severity of hemiplegic attacks and worse clinical cases, for example, increased frequency of status epilepticus and/or apnea attacks needing emergent care3). Because we could not get flunarizine in Japan, all family members who wanted to continue flunarizine had to import it individually through an import agency.
Notably, family members and I asked several Japanese pharmaceutical companies to produce flunarizine again. All companies asked refused our request because (1) the subject number was too small, and (2) although the clinical trial was expensive, flunarizine was relatively low price.
Furthermore, in 2012, we analyzed the ATP1A3 mutation in many Japanese patients with AHC and researched their clinical courses and flunarizine usage. In some patients with AHC, especially with the E815K variant, clinical deterioration occurred after flunarizine discontinuation. However, patients with AHC with the D801N variant showed no clinical deterioration after flunarizine discontinuation4). Thus, flunarizine might have different effects on ATP1A3 variants, or we saw natural clinical courses in patients with AHC.
Finally, in 2022, we re-investigated flunarizine usage in patients with AHC in Japan. Twelve patients use flunarizine in 33 members of Japan family association of AHC. Additionally, all patients get flunarizine through individual import from foreign countries. The efficacy of flunarizine is unclear for most patients; therefore, we do not strongly recommend flunarizine use for patients with AHC without the E815K variant.
Dr Tsveta Schyns-Liharska, PhD
Tsveta has academic background with PhD in molecular genetics. She is parent of a child with rare neurological disease, caused by ATP1A3 gene mutation. This motivated her to set up the EU-funded research projects ENRAH (from 2003) and nEUroped (2010-2014), and the ATP1A3disease Symposium (since 2012), which became fundamental to advance understanding of ATP1A3 diseases. In addition to these projects, Tsveta served as member of the Paediatric Committee at the European Medicines Agency (2008-2017) and works with the European Commission as reviewer of EU grant proposals (since 2008) and Blue Book trainee (2019-2020) at DG Research and Innovation. For her work for the rare disease community, Tsveta received the EURORDIS Black Pearl award in 2016.
The ATP1A3 in Disease Symposium Standing Committee
Professor Sanjay Sisodiya
Sanjay Sisodiya is Professor of Neurology at UCL Institute of Neurology and Honorary Consultant Neurologist at the National Hospital for Neurology and Neurosurgery and the Epilepsy Society. His key interests are in epilepsy, difficult-to-treat epilepsy, phenotyping in epilepsy and genetic research into epilepsy, including its causes, pathology, phenotype development and treatments. He was chair of the Association of British Neurologist Epilepsy Advisory Group from 2015-2021, providing national guidance on issues such as medicinal cannabis and valproate usage in women of childbearing age. He leads a number of international projects including EpiPGX, ENIGMA-Epilepsy and EpilepsyClimateChange. He is Deputy Director for Sustainability and Climate Change at the UCL Queen Square Institute of Neurology, and Chair of the International League Against Epilepsy Climate Change Commission. He is a scientific advisor for AHC UK
Debate: What’s in a name? How should AHC be named and classified for families, clinical practice, and research?
The term ‘alternating hemiplegia of childhood’ has been used for many years. Since its invention, we have learnt much more this, and related, conditions, including their genetic basis, underlying mechanisms, clinical presentations and features, and longer-term outcomes. Words, names and labels are crucially important for many reasons. In this session, we will discuss whether current names are appropriate, or need to be reconsidered.
Mr Alexander Sousa
Alexander A. Sousa is a Harvard University PhD candidate in the laboratory of Dr. David R. Liu at the Broad Institute of Harvard and MIT and is a National Science Foundation Graduate Research Fellow. He completed his undergraduate degree in Biology at Northeastern University in 2016 and simultaneously trained as an undergraduate researcher at Editas Medicine from 2014-2016. His post-undergraduate work was completed in the laboratory of Dr. J. Keith Joung at Massachusetts General Hospital and Harvard Medical School, where he worked as a research technologist investigating Cas12a CRISPR nuclease protein engineering and the development of improved-specificity CRISPR nucleases. As a Harvard graduate student in the Liu lab his research is focused on the development of new genome editing technologies and their application for the correction of the underlying pathogenic mutations of monogenic diseases. “ATP1A3 gene editing: Using CRISPR for ATP1A3 diseases”.
ATP1A3 gene editing: Using CRISPR for ATP1A3 diseases
CRISPR-based precision gene editing technologies hold broad therapeutic promise by offering direct correction of disease-associated genetic variants. Prime editing is a “search and replace” precision gene editing technology that enables the installation of all single base pair substitutions and the creation of sequence-defined small insertions and deletions, without requiring deleterious double-stranded DNA breaks or donor DNA templates. Here, we provide an update on our progress to develop a prime editing strategy to correct the Atp1a3 D801N mutation found in a mouse model of Alternating Hemiplegia of Childhood (AHC). By leveraging recent prime editing advances from our research group, we have used cell-based experiments to develop a prime editing strategy that can efficiently correct the Atp1a3 D801N mutation while minimizing the generation of undesirable editing byproducts. The development of this optimized prime editing strategy is a key step towards our goal of treating a post-natal AHC animal model with prime editing reagents to evaluate editing-related survival enhancements and AHC disease mitigation.
Lay summary
Prime editing is a molecular tool that can be programmed to correct mistakes in DNA, similar to the “search-and-replace” function of a word processor. In principle, prime editing should be able to correct the Atp1a3 D801N mutation that causes Alternating Hemiplegia of Childhood (AHC) disease in a mouse model of AHC. Our ultimate research goal is to determine if we can improve the mouse model’s survival and/or mitigate its AHC disease by correcting this mutation with prime editing. Using prime editing to successfully treat AHC disease in these mice would suggest that gene editing may hold promise as a future therapeutic to treat AHC.
Here, we discuss our efforts to develop a prime editing strategy that is designed to correct the Atp1a3 D801N mutation found in an AHC mouse model. By combining recent improvements to the prime editing technology, we have developed a prime editing strategy that efficiently corrects the Atp1a3 D801N mutation in mouse cells. This is an important step towards our future experiments in mice.
Dr Agnieszka Stępień
Physiotherapist. Lecturer at the Faculty of Rehabilitation, Józef Pilsudski University of Physical Education in Warsaw, Poland. The doctoral theses in the field of spine biomechanics in children and adolescents with idiopathic scoliosis. Clinical experience in the treatment of patients with neuromuscular diseases and other rare diseases.
President of the Polish Physiotherapy Association (2017-2022). Member of the Unit of the National Program for Rare Diseases in Poland, Ministry of Health (2016-2017). Member of the Unit for Diagnostics and Conservative Treatment of Idiopathic Scoliosis, Polish Academy of Sciences (2016-2020). Advanced Instructor of the International Proprioceptive Neuromuscular Facilitation Association (IPNFA), member of the Research Committee IPNFA. Speaker at numerous scientific conferences and workshops in Poland and abroad.
The role of the physiotherapist in the multi-disciplinary team
Alternating hemiplegia of childhood (AHC) is a rare neurological disease characterized by temporary paralysis and other paroxysmal symptoms such as respiratory disorders, dysarthria, dysphagia, facial dyskinesia, as well as autonomic disturbances. The disease causes many limitations in everyday functioning and social life. Physiotherapy is an important part of the treatment of AHC patients.
The main goal of physiotherapy is to improve the quality of life of people with AHC, as well as substantive support for families. So far, no detailed standards of physiotherapy have been developed. There is a little scientific knowledge in this field. The low incidence of AHC and various clinical course of the disease are the main reasons.
Therefore, physiotherapy should be planned on the basis of a detailed examination, according to the established goal of therapy. Most often, the main goal is to improve motor skills. Usually, disturbances of selective hand movements and gait are reported by patients and their families. Disorders of jumping, running and eye-hand coordination have also been observed in this group.
Physiotherapy includes many therapeutic procedures to improve motor skills. Physiotherapeutic interventions can also improve cardiovascular function (pulse rate, respiratory parameters, respiratory muscle strength, chest elasticity, tension of secondary respiratory muscles), speech quality (speech volume, lip and tongue movements, jaw movements), swallowing (muscle strength, body alignment), facial movements and reduce pain. Regularly performed exercises influence structures and functions of the musculoskeletal system (postural muscles strength, alignment of the spine and limbs, shape of the chest). Physiotherapists should cooperate with medical specialists, including paediatricians, orthopaedists, cardiologists, speech therapists, psychotherapists, dentists and others. The effectiveness of physiotherapy should be assessed in future studies.
Ms Sonal Sumaria
Expert by experience
Lived experience CAPOS
Professor Kathleen Sweadner
As a graduate student in 1977, Kathleen Sweadner discovered that there was a second form of Na,K-ATPase alpha subunit (now known as ATP1A3) in brain. She went on to show that it had different properties from the known Na,K-ATPase in kidney, ATP1A1, and from the third isoform, ATP1A2. Her lab determined cell-type specificity for their expression in brain and retina; their developmental regulation in retina and heart; and the regulation of Na,K-ATPase activity by protein kinases and a family of small regulatory proteins, the FXYD proteins. She and Elena Arystarkhova have been doing laboratory investigation of human mutations of ATP1A3 for 20 years. Initially they experimentally tested the pathogenicity of new variants, but increasingly on how different mutations produce different clinical phenotypes. This led to the appreciation that pathogenicity often entails more than just impairment of pump activity. She is also an avid student of crystal and cryoEM atomic structures of Na,K-ATPase for the value they bring to understanding mutation consequences.
ATP1A3 disease –phenotypic description to gene discovery. Cellular clues to ATP1A3 syndromes
The discovery of mutations in ATP1A3 was made possible by the passion of neurologists, Dr.s Allison Brashear and Kathryn Swoboda, who tirelessly gathered information on groups of patients that shared symptoms. Their work made it possible for geneticists to apply the best technology of that time to pin down the gene. For RDP, that entailed building large family pedigrees so that Dr. Laurie Ozelius could narrow down a chromosomal location and then painstakingly sequence candidate genes. For AHC, it was then-new whole exome sequencing by Erin Heinzen and David Goldstein. It succeeded because Dr. Swoboda had selected patients so carefully, the first ones tested all had the same mutation. After that, whole exome and whole genome sequencing has yielded diagnoses for many patients and a wide range of phenotypes.
The symptoms of ATP1A3 mutations are on a continuum in the sense that certain features show up frequently across a range of patients. We can think of these as weak points in the nervous system where a loss of Na,K-ATPase activity produces diagnosable symptoms, usually motor symptoms. This continuum is superimposed on discrete syndromes that have a strong bias to be associated with particular mutations. These syndromes are 1) prenatal brain malformation (a defect in cortical layering); 2) perinatal or progressive microcephaly, or later atrophy (due to neuron loss); 3) intractable epilepsy at birth; 4) AHC with paroxysmal symptoms; 5) CAPOS, where fever-induced ataxia is mysteriously followed later by loss of sight and hearing; 6) fever-induced ataxia/weakness in R756 mutations; and 7) RDP.
A core feature is that virtually all ATP1A3 mutations are heterozygous, yet the genetic data show few stop codons and deletions that could cause haploinsufficiency. There is also a lack of homozygous mutations with partial activity loss. This is not the case in all P-type ATPases: Wilson’s disease for example is recessive. We and others have shown that for ATP1A3, disease severity does not correlate with whether some Na,K-ATPase activity remains. Our lab has undertaken to compare what happens in isogenic cell lines that express ATP1A3 mutations typical of different syndromes. So far, three distinct cell biological responses can be associated with syndromes with neuron loss, with R756 mutations, or with AHC, that we have not seen with RDP mutations. How the cell responds to the presence of a particular mutation is part of what we need to know to make meaningful progress.
Dr Danilo Tiziano
Catholic University of the Sacred Heart, Milan, Italy
Update from the TREAT AHC research study: what drugs are being tried?
Over the last few years, we have developed and characterized a cellular model of AHC, based on a human neuroblastoma cell line (SH-SY5Y). SH-SY5H cells display a neuronal-like phenotype and express the endogenous ATP1A3 gene. To characterize the phenotype of naïve SH-SY5Y, cells were differentiated with retinoic acid followed by neurobasal medium/B27 supplement. This protocol led to the development of neuron-like cells showing Ca2+ transients, trains of action potentials, and the expression of neuronal markers.
The AHC model was developed by permanently transfecting SH-SY5Y with plasmids bearing the ATP1A3 variants more commonly found in patients: Glu815Lys, Asp801Asn, Asp801Tyr or Gly947Arg. Mixed cell populations underwent clonal selection. The expression levels of endogenous and mutated ATP1A3 mRNA were determined by absolute real-time PCR; we prioritized clones having mutated/wt cDNA ratio close to 1:1.
The main phenotypic characteristics of mutated cells were: intracellular accumulation of Na+ and Ca2+, significant reduction of cytoplasmic pH, membrane depolarization, and apoptosis during differentiation. We are currently addressing the molecular pathophysiology by transcriptome analysis. Intriguingly, the mutated allele was silenced over in vitro passages, likely due to epigenetic mechanisms, although the treatment with de-methylating agents did not restore the expression of the transgene. We also characterized the gene expression profile of the mutated cell line by whole transcriptome analysis and found a high representation of genes belonging to different DNA repair pathways, to oxidative phosphorylation and several neurodegenerative pathways.
Our cell model presents a striking phenotype, easily traceable evaluable by high-content screening platforms. We have analyzed 551 safe-in-men compounds for their ability to restore Na+ and Ca2+ levels in cells expressing the Asp801Asn variant. Twenty-seven compounds were prioritized for their ability to reduce Na+ levels in mutated cells only. Of these, 3 were considered particularly promising and were also tested in an animal model of AHC, bearing the D801N mutation. We are currently setting a phase I clinical trial to test the safety of the two most promising molecules. The identification of an industrial partner is crucial to proceed further with drug development.
This work was supported by AISEA, La Marato de TV3, AESHA, Duke University.
Dr Don Urquhart
Don Urquhart is a Consultant and Honorary Reader in Paediatric Respiratory Medicine at the Royal
Hospital for Children and Young People and the University of Edinburgh. Don is a clinician and researcher whose main research interests are sleep medicine and exercise physiology. He has an MD from UCL studying the effects hypoxaemia on sleep and exercise in children with Cystic Fibrosis. In addition, Don has undertaken work on sleep in epilepsy, has completed a number of exercise studies in children with cystic fibrosis, has been principal investigator on several pharmaceutical trials, and has contributed to the writing of national and international guidelines on exercise testing. Don is a previous chair of the British Paediatric Sleep Society.
The need for an MDT to manage AHC – how should this be composed? Respiratory
Alternating hemiplegia of childhood (AHC) is associated with the potential for respiratory problems. Impaired airway clearance may ensue as a result of muscle weakness and chest wall dystonia, increasing the likelihood of respiratory infection; whilst bulbar involvement may be associated with aspiration. In addition, a variety of sleep problems are associated with AHC including sleep onset difficulties, sleep maintenance issues and sleep-disordered breathing. This talk will aim to provide a framework for the evaluation and investigation of respiratory and sleep issues in AHC.
The aims of the session are:
- To discuss the effects of AHC on the respiratory system
- To discuss a structure for the evaluation of respiratory health in AHC
- To provide an overview of sleep problems and sleep-disordered breathing in AHC
- To briefly describe sleep diagnostics and what they measure
- To discuss the importance of a child-centred management plan to optimise respiratory health and manage AHC crises
Professor Arn Van den Maagdenberg
Arn M.J.M. van den Maagdenberg is full professor, neurogeneticist and neurobiologist, and holds a Chair in Functional and Molecular Neurogenetics at the Leiden University Medical Center in Leiden, the Netherlands with affiliations at both the Departments of Human Genetics and Neurology. He studied biology at the University of Nijmegen and received his PhD in 1993 at Leiden University for which he investigated molecular mechanisms of cardiovascular disease in patients and transgenic mouse models. In 1998, he joined the Leiden migraine group and became full professor in 2011. His genetic and functional research focuses on elucidating disease mechanisms of episodic neurological diseases with a focus on migraine, and comorbid disorders epilepsy and stroke. A large part of his research is aimed at the identification of mutations causing monogenic brain disorders, such as hemiplegic migraine, and elucidating their functional consequences by applying neurobiological approaches in transgenic mice that carry pathogenic human gene mutations. He has been an active member of research efforts to find the ATP1A3 gene in alternating hemiplegia of childhood.
What does it mean to have a ‘broken’ ATP1A3 pump?
Many patients with alternating hemiplegia of childhood (AHC) have a mutation in the ATP1A3 gene that causes malfunction of a pump. In this talk I would like to take a step back and ask the simplified question “what does it mean to have a broken ATP1A3 pump?”. It intends to review knowledge we have about the ATP1A3 pump in AHC using complex data from researchers in the field discussed in more laymen terms. Hopefully, the presentation will aid the non-scientists to grasp a bit better what it is researchers are trying to achieve. The information may also be of help to understand a bit easier what will be discussed in the remainder of the scientific symposium. The ATP1A3 gene encodes for a subunit of a sodium-potassium ATPase, which is a protein complex with an important function in the brain. Using the rather simplistic analogy when one is confronted with a broken water pump in one’s house, one could have the straightforward idea: “so fix what is broken”. What actually are some of the challenges that have prevented researchers of reaching this goal? This talk will try to explain in easy terms what an ATP1A3 pump does, what is wrong with the pump in patients with AHC, and how researchers have come to their conclusions. The following questions will be addressed. Where are the broken pumps located? What are – in general terms – the consequences of a broken pump? How can they lead to the complex clinical phenotype? Can something be done about a broken pump? And finally, are there lessons to learn from other examples, in this case hemiplegic migraine, where there is also a dysfunction of a sodium-potassium ATPase, so a broken pump, but in that case a broken ATP1A2 pump?
Dr Aikaterini Vezyroglou
Dr Aikaterini Vezyroglou is a Paediatric Neurologist working at the UCL GOSH Institute of Child Health in London. After studying Medicine in Thessaloniki, Greece, she completed her Paediatric Neurology Training in Cologne, Germany and then joined Great Ormond Street Hospital in 2015 for her Clinical Fellowship in Complex Childhood Epilepsy. She is currently completing her PhD on ATP1A3-related disease.
Addressing the genotype-phenotype correlation in AHC and ATP1A3 diseases
Throughout the last 20 years, pathogenic variants in ATP1A3 have been discovered to cause an ever-expanding range of rare neurological phenotypes, affecting both children and adults. These phenotypes include, but are not limited to, Rapid-Onset Dystonia Parkinsonism (RDP), Alternating Hemiplegia of Childhood (AHC), Cerebellar ataxia, Areflexia, Pes cavus, Optic atrophy, Sensorineural deafness (CAPOS) syndrome, Early Infancy Epileptic Encephalopathy (EIEE), sometimes accompanied by polymicrogyria (PMG) and Relapsing Encephalopathy with Cerebellar Ataxia (RECA). As these conditions are extremely varied in phenotype and prognosis, being able to predict the phenotype at the time of genetic diagnosis would be very useful to council families, as well as anticipate treatment strategies and future complications.
There is some evidence for genotype-phenotype correlation in ATP1A3-related conditions. In recently published work we reviewed the literature for all pathogenic ATP1A3 variants published between 2004 and 2021. 168 pathogenic ATP1A3 variants had been published in 1108 reported individuals. Two thirds (65.2%) of the cases (n=721) carried one of eight variants. These were p.Asp801Asn)(n=293), p.Glu815Lys (n=180) and p.Gly947Arg) (n=77) the three most common variants associated with AHC, p.Glu818Lys (n=53), the single variant associated with CAPOS, variants p.Thr613Met (n=49) and p.Ile758Ser (n=27), the most common genotypes associated with RDP, and finally p.Arg756His/Cys (n=26/16) causing RECA.
This phenotype-genotype correlation can be further broken down for the 3 most common AHC mutations. In publications by Sasaki et al., Panagiotakaki et al. and Uchitel et al. patients with variant p.Glu815Lys were consistently shown to be more severely affected than patients with variant p.Asp801Asn. Patients with variant p.Gly947Arg had the mildest phenotype.
However, we do not know whether a similar correlation between phenotype and genotype exists for the remaining 160 variants, as for most only 1-2 patients have been described so far. This might be due to them being very rare or maybe the patients are not recognized as having an ATP1A3-related condition, due to phenotypic variability. To address this question, we launched the VARIA-ATP1A3 study through the International AHC Research Consortium (IAHCRC). We propose an online survey of phenotypic characteristics of patients carrying a rare pathogenic/ likely pathogenic ATP1A3 variant, in order to:
- establish the actual width of the ATP1A3-related disease spectrum;
- establish whether genotype/phenotype correlation exists for the rarer ATP1A3 variants.
- establish a registry of patients with rarer variants.
The study is recruiting centres regardless of whether they are part of the IAHCRC. Please get in touch if you are interested.
Professor Bente Vilsen
Bente Vilsen, DMSc, Professor of Physiology, Aarhus University, Denmark, since 2006. Author of 127 papers in international journals on molecular structure, mechanism, and pathophysiology of ion pumps, in particular the Na,K,-pump. Of these, 15 deal with Na,K-pump mutations causing neurological disease.
Rescue of Na+/K+-ATPase mutational effects by secondary mutation: Perspective for future pharmaceutical intervention in ATP1A3 neurological disease.
Neurological disease mutations of the Na,K-ATPase may lead to disturbance of function and/or protein misfolding and reduced expression of active enzyme. Some of those disease mutations that primarily disturb Na,K-ATPase function act by reducing the affinity for Na+, thereby decreasing the transport activity in vivo. We previously found that the second-site mutation E314D rescues the reduced Na+ affinity and activity of the D928N α1-mutant, where Na+ site III is disturbed (1,2). A successful strategy for pharmacological intervention in neurological disease might be to design a pharmaceutical that interferes mechanistically with the Na,K-ATPase in the same way as the rescue mutation by binding in the enzyme domain where the rescue mutation is located. To gain understanding of the molecular mechanism underlying the rescue effect and its applicability to relieve neurological disease, we have carried out a series of experiments exploring the rescue effects on α3-disease mutants and mutants with distinct disturbance of either site I, II, or III. Our findings have led to a hypothesis explaining the rescue effect, and during the course of these experiments we have discovered new rescue mutations that are more efficient than the original one.
Dr Suellen Walker
Suellen Walker is Professor of Paediatric Anaesthesia and Pain Medicine, UCL GOS Institute of Child Health and Great Ormond Street Hospital, London, UK. Following her medical degree, she completed a Masters in Medicine in Pain Management, MSc in Neuroscience, and PhD in developmental neurobiology of pain. She was a Foundation Diplomate of the Faculty of Pain Medicine, Australian and New Zealand College of Anaesthetists when it was established in 1999 and was Elected to Fellowship of the Faculty of Pain Medicine, Royal College of Anaesthetists, UK in 2016. Her clinical activities include acute and chronic paediatric pain management, and her research interests as Principal Investigator of the Paediatric Pain Research Group include potential long-term effects of early life pain and injury, and neuropathic pain in children.
The need for an MDT to manage AHC – how should this be composed? Pain Medicine
POSTERS
Please take the opportunity to review the posters displayed in the main foyer of the conference centre. Abstracts are available.
- Structural connectivity in Alternating Hemiplegia of Childhood1.
Mariasavina Severino MD*1, Livia Pisciotta MD*3, Domenico Tortora MD*, PhD1, Ramona Cordani MD2,5, Michela Stagnaro MD, PhD5, Benedetta Toselli PhD4, Marcella Gherzi MD5, Rosella Tro PhD4, Marco Fato4, Andrea Rossi MD1,6, Lino Nobili MD, PhD2,5, Elisa De Grandis MD, PhD2,5 and The IBAHC Consortium.
Objective: to evaluate the structural connectivity of subjects with alternating hemiplegia of childhood (AHC) by using constrained spherical deconvolution tractography and connectome analysis.
Methods: In this prospective single-center study, 10 AHC subjects (mean age 23.2 years) and 20 age and sex-matched controls were studied with 3DT1-weighted MR imaging and high angular resolution diffusion imaging at 3T. Data obtained from structural connectivity analysis, including summary global and local network metrics, modularity analysis, and network measures, were correlated with cognitive and motor impairment using the Wechsler scales, Vineland Adaptive Behavior Scales-II, International Cooperative Ataxia Rating Scale and Movement and Disability sub-scales of Burke-Fahn-Marsden Dystonia Rating Scale.
Results: At a global level, patients with AHC had a less integrated structural connectivity, with higher characteristic path length (p=.044) and lower global efficiency (p=.041) compared to normal controls. The mean degree (p=.039) and mean normalized betweenness (p=.044) were also reduced compared to normal controls. No significant differences regarding segregation or modularity metrics were found. The Network-based statistics identified a single subnetwork of decreased connectivity in AHC patients compared with controls involving nodes in the basal ganglia region, and in frontal and insular lobes of both hemispheres (p=.016). At a nodal level, there were widespread differences in degree between AHC patients and controls (21 out of the 84 nodes), mainly located in frontal, limbic, temporal, and occipital lobes and at level of basal ganglia. Lower degree of the left medial orbito-frontal (r=.650, p=.042) and superior temporal nodes (r=.762, p=.01) and right caudate (r=.737, p=.015) and hippocampus (r=.643, p=.045) correlated with lower working memory indices.
Conclusion: These results suggest that the wiring of the structural brain network of AHC patients is altered relative to healthy controls in such a way that it allows for less integration of information processing with potential disruption of circuits involved in working memory.
- FXYD6 Dimerization Dysregulates NKA in Neurological Diseases2
John Q. Yap, Taylor A. Phillips, Jaroslava Seflova, Jacob S. Cunningham, Carson S. Miller, Seth L. Robia
The Na+/K+ ATPase (NKA) is an ATP dependent transporter responsible for maintaining Na+ and K+ electrochemical gradients. In the brain, NKA activity must be tightly regulated to mediate several cellular processes, including synaptic transmission and generation of action potentials. It has been shown that NKA in the brain is inhibited by FXYD6, a member of the FXYD protein family. FXYD6 has been shown to participate in neuronal excitability, but also plays a role in neurological diseases, including schizophrenia. The mechanisms by which FXYD6 regulates NKA in the healthy and diseased brain are not well established. In this study, western blotting revealed that FXYD6 can form dimers in transfected HEK cells. This is an important phenomenon since FXYD6 binds to NKA as monomers. Because FXYD6 contains three cysteine residues (C9, C60 and C62), we hypothesized that FXYD6 dimerization occurs due to oxidative crosslinking of cysteine residues. To test our hypothesis, we exposed cells expressing FXYD6 to the oxidizing agents diamide or H2O2. Oxidation of FXYD6 increased dimerization, as measured by fluorescence resonance energy transfer (FRET) and western blotting, and this was reversed by subsequent addition of the reducing agent dithiothreitol (DTT). To determine which cysteine residue(s) were responsible for FXYD6 dimerization, we mutated individual cysteine residues and measured FRET between FXYD6 mutants. Our results showed that mutation of each cysteine residue decreased FRET between FXYD6 proteins, suggesting that all three cysteines contribute to FXYD6 dimerization. Additionally, mutation of individual FXYD6 cysteines increased FRET between FXYD6 and NKA, suggesting that FXYD6 binds to NKA as monomers. These results suggest that FXYD6 binds NKA as monomers in the healthy brain. However, in neurological diseases where there is increased oxidative stress, FXYD6 dimerization sequesters monomeric FXYD6 away from NKA, resulting in NKA dysregulation.
- Development and evaluation of the usefulness of a smart phone and web accessible electronic diary (e-Diary) in Alternating Hemiplegia of Childhood3.
Maria T. Papadopoulou1, Marion Comajuan1, Lyndsey Prange2, Jennifer Anticona Huaynate3, Aikaterini Vezyroglou4,5, Michela Stagnaro6, Andrey Megvinov7, Carmen Fons3, Elisa De Grandis6, Maria Sentmanat2, Simona Balestrini8, Jeffrey Wuchich9, Sigurður Jóhannesson10, The IAHCRC OBSERV-AHC Study Group, Alexis Arzimanoglou1,3, Mohamad A. Mikati2, Rosaria Vavassori7,11,12, Eleni Panagiotakaki1
Background: Alternating Hemiplegia of Childhood (AHC) presents differently amongst patients, with varying levels of severity that is in part associated with the type and frequency of paroxysmal events. Disease- specific tools for AHC patients and caregivers in order to record spells with reliability and ease are lacking.
Objectives: An electronic version of the custom-designed daily event calendar for spells (e-Diary) used for the needs of the OBSERV-AHC Study (carried out by a number of centers of the IAHCRC Consortium) was developed and proposed to AHC patients and their caregivers. We aimed to evaluate whether the e-Diary tool could facilitate the event recording.
Methods: Participants of four European sites of the OBSERV-AHC study (France, Italy, Spain, United Kingdom) were offered the possibility of usage of a smart phone and web accessible electronic diary (e-Diary) to record their patients’ events in the IAHCRC-CLOUD Platform, to be used for the OBSERV-AHC Study. The e-Diary was based on the paper version of the event calendar that was developed by a panel of caregivers and experts in the field. Mandatory fields for completion included the date, time, duration and type of the recorded spells. An explicit presentation of the e-Diary was done by a trained physician/ researcher during an in-person or video meeting with caregivers, along with an illustrative training on the identification of the type of the spells when possible, followed by an online registration and personal credentials creation on the IAHCRC-CLOUD Platform for all caregivers that expressed interest for using the e-Diary. The research team had access to the electronic event records on the Platform but follow-up communication regarding the e-Diary was available only upon caregivers’ specific request. Caregivers’ feedback regarding the usefulness of the e-Diary was evaluated via a telephone survey conducted during March 2022. IBM SPSS 21 was used for quantitative data analysis and qualitative analysis for open- questions data.
Results: Survey responses from caregivers of thirty-three AHC patients [(mean age: 13.5 years (range: 1.8- 41.3 years, SD: 1.8); 51.6% male] that were offered the possibility of the e-Diary usage were collected. Half of the respondents (N=17) used the e-Diary with a mean time of usage of 10.47 months (range 1- 24 months, SD: 1.96). Log rank tests showed no differences in the time of usage of the e-Diary when patients were grouped according to the frequency of paroxysmal events and to the delivery of a video training before the e-Diary usage. The users mostly appreciated: the ease of the reporting (27.6%), the feeling of contribution to better understanding of the disease (20.7%), the environment of the application (13.7%), the fact that the records were directly available to researchers/clinicians (13.7%), and the feeling of engagement to the research procedure (13.7%). The main reasons for no/end of usage were lack of practicality (21.2%) and time (21.2%). The tool was considered a good initiative by 87% of the respondents; 97% of caregivers (including those that did not use the e-Diary in the context of this study) responded that they could engage to use it in the future (69.7% for more than 2 years) with some modifications.
Conclusions: An electronic version of a costumed to AHC calendar to record spells could be useful for caregivers. Modifications of the e-Diary according to caregivers’ suggestions are essential for ensuring its sustainable utilization in natural history studies and clinical trials, as well as for better care of patients.
Acknowledgements: We thank the following AHC patient organizations for their support of the OBSERV-AHC Study, with this Event Calendar sub-project, and of the IAHCRC-CLOUD Platform, serving the data collection for the OBSERV-AHC Study, the Videolibrary and the Training Procedure and the online Event Calendar (e-Diary): USA CureAHC Foundation, French (AFHA), Icelandic (AHC Samtökin), Dutch (AHC Vereniging Nederland), Spanish (AESHA), UK (AHC UK), German (AHC18+ e.V.), Polish (AHC-PL) and Italian (AISEA) Associations.
- Sodium pump regulation in health and disease4
Jaroslava Seflova1, Nima R. Habibi2, Ryan Sweazey3, John Q. Yap1, Sean R. Cleary1, Xuan Fang1, Peter Kekenes-Huskey1, L. Michel Espinoza-Fonseca4, Pablo Artigas3, Julie Bossuyt2, and Seth L. Robia1
The sodium-potassium ATPase (NKA) is the ion motive ATP-dependent transporter that establishes Na+ and K+ gradients across the cell membrane, facilitating many physiological processes. In the heart, NKA activity is regulated by phospholemman (PLM, FXYD1), a member of the FXYD protein family. PLM inhibits NKA by reducing the pump’s apparent affinity of the pump for Na+, which is relieved by PLM phosphorylation. In this study, we used time-correlated single-photon counting, FRET-microscopy, and molecular dynamics simulations to investigate the structure, stoichiometry, and affinity of the NKA-PLM regulatory complex. We observed a concentration-dependent association of the subunits of the NKA-PLM regulatory complex. We observed avid association of the catalytic a subunit and essential b subunit, followed by lower affinity a-a and a-PLM interactions. The data suggest a regulatory complex composed of two a subunits associated with two b subunits and decorated with two PLM regulatory subunits. We propose that a-a subunit interactions support conformational coupling of the catalytic subunits, which enhances NKA turnover rate which is important for cells with high ion transport demands such as kidneys or heart. Protein-protein docking and molecular dynamics simulations generated a structural model of the regulatory complex. This model was characterized by contacts between cytoplasmic domains of a subunits, between b subunit extracellular domains, and between the highly-conserved a subunits’ M3 helix and the N-termini of opposing β subunit. Furthermore, the mutations in M3 were previously associated with alternating hemiplegia of childhood, renal hypomagnesemia, and familial hemiplegic migraine type 2. Specifically, we were able to associate the mutation of residue G301 with a decrease in the dimer formation and leak current through the pump causing cell depolarization. Together, the data provide an insight into the quaternary structure of the NKA-PLM regulatory complex with a stoichiometry of (ab-PLM)2 and represent a collaborative platform for the study of NKA structure-to-function relationships.
- ATP1alpha3 subunit controls axon navigation in embryonic spinal cord5
Sophie Calvet1, Sarah Dinvaut1, Olivier Pascual1, Rosaria Ferrigno2, Frédéric Moret1, Julien Falk1*&,Valérie Castellani1*&
Mutations of the Na+/K+ATPase alpha3 (ATP1A3) subunit are responsible of early neuropediatricdisorders that can associate with developmental alteration of brain architecture. We investigatedwhether ATP1A3 could contribute to the formation of neuronal projections. Consistently with an earlyrole of ATP1A3 during neurodevelopment, we found that it is expressed in the spinal cord of chickembryos, notably in dorsal interneurons (DINs) when they form their axons. Furthermore, we coulddetect alpha3 subunit in the developing axons of DINs. We then performed in vivo ATP1A3 knock-down and overexpression of the wild-type ATP1A3 or the transport-deficient E815K mutant. Weanalyzed DIN axons trajectories in cleared whole mount embryos and found that all ATPA3manipulations altered axon trajectories. To our surprise, inhibition of Na+/K+ATPase did not alter theresponse to biochemical cues used by these axons to navigate. Rather, we found that Na+/K+ATPasessupport electric field (EF) directed migration. As we could measure EFs in the developing chick spinalcord and show that EFs orient DIN axons in vitro, we tested the axon response to EFs afterNa+/K+ATPases inhibition and ATP1A3 knock-down. We found that both manipulations inhibit EF-induced orientation of DIN axons supporting a key role of alpha3 subunit in mediating axon responseto EFs. In vivo perturbations of spinal cord EF impaired axon navigation also supporting that ATP1A3-mediated orientation by EF contributes to control axon development. Noteworthy, expression of theATP1A3 bearing the disease-causing mutation E815K strongly impacted DIN axons navigation. Thus,altogether, our results support a role of ATP1A3 during the formation of neuronal circuits whosealterations may contribute to the etiology of some ATP1A3 syndromes.
- Maturation of GABAergic neurotransmission is impaired in AHC neurons6
Christine Q. Simmons, Christopher H. Thompson, Alfred L. George, Jr.
Aside from the acute transient neurological symptoms that characterize alternating hemiplegia of childhood associated with ATP1A3 pathogenic variants, there are long-term manifestations including delayed neurodevelopment. One cellular mechanism that may contribute to delayed neurodevelopment is impaired maturation of GABAergic neurotransmission. In developing neurons, the neurotransmitter GABA elicits depolarization of the post-synaptic membrane owing to high intracellular chloride ion (Cl-) concentration, which favors Cl- efflux through activated ionotropic GABA receptors. By contrast in mature neurons, expression of a potassium chloride (KCl) co-transporter (KCC2) maintains intracellular Cl- concentration at a lower level such that GABA receptor activation produces Cl- influx and hyperpolarization of the post-synaptic membrane, which inhibits neuronal excitability. We hypothesized that the blunted transmembrane K+ gradient resulting from impaired Na/K-ATPase ion pumping reduces the driving force for K+ efflux through KCl co-transport mediated by KCC2. Secondarily, disruption of KCC2-mediated lowering of intracellular Cl- during neuronal maturation will affect the transition of intracellular Cl- concentration and disrupt the normal developmental switch of GABAergic neurotransmission from excitatory to inhibitory (‘GABA switch’). We tested this hypothesis using human cortical neurons differentiated from patient-specific induced pluripotent stem cells (iPSC). For these experiments we used neurons differentiated from iPSC lines with either ATP1A3-G947R or ATP1A3-L839P and corresponding isogenic mutation-corrected control iPSC lines engineered using CRISPR/Cas9 genome editing. To investigate the timing of the GABA switch, we monitored the reversal potential of neurons during exposure to extracellular GABA (EGABA). To avoid disruption of the transmembrane Cl- gradient during these experiments, we employed gramicidin perforated patch clamp recording. EGABA was measured in voltage clamp mode. GABA receptor mediated currents were elicited by serial applications of 100 µM GABA while cells were clamped from -90 to -20 in 10 mV increments. EGABA was determined as the x-intercept from a linear regression of the resulting current-voltage relationship. We measured EGABA at 6 time points from post-differentiation day 19 to 65 obtained from the isogenic neuron pairs. We determined the percentage of cells at each time point that exhibited an EGABA of -60 mV or more hyperpolarized as an index of the proportion that had achieved the GABA switch. We observed that ATP1A3 mutant neurons exhibit a significantly later GABA switch as compared with neurons from isogenic mutation-corrected iPSC lines. This delay in GABA switch was also evident during microelectrode array (MEA) recording of mixed cultures of iPSC-derived cortical excitatory and inhibitory neurons. Our findings support the hypothesis that ATP1A3 pathogenic variants are associated with impaired maturation of GABAergic neurotransmission in human iPSC-neurons. This phenomenon may help explain delayed neurodevelopment in AHC.
- Development and Reliability Testing of a Calendar Method to Record Spells in Alternating Hemiplegia of Childhood7
Maria Sentmanat1, Lyndsey Prange1, Maria T. Papadopoulou2, Carmen Fons3, Elisa De Grandis4, Simona Balestrini5, Aikaterini Vezyroglou6, Jeffrey Wuchich7, Sigurður Jóhannesson8, The IAHCRC OBSERV-AHC Study Group, Eleni Panagiotakaki2,9, Rosaria Vavassori10,11, Mohamad A. Mikati1
Background: Alternating Hemiplegia of Childhood (AHC) presents differently amongst patients, with varying levels of severity and types of spells. Developing reliable methods to record such spells is essential for natural history studies, clinical trials as well as for better care of patients.
Objectives: To test the following hypotheses: 1) Use of Video library of AHC type spells for training results in improvement of participants’ ability to reliably identify AHC spells. 2) A custom designed daily event calendar for spells with weekly reviews between parents and providers results in overall reliable documentation of such events. In addition, the goal of the calendar was also for it to be used for the OBSERV-AHC Study carried out by a number of centers of the IAHCRC Consortium.
Methods: Video Library Training Study: A video library of AHC type spells including separate illustrative (for caregiver training) and test cases (to evaluate ability of caregivers to identify spells before and after the training) was developed online, in the IAHCRC-CLOUD Platform, and agreed on by 5 experts in the field. In addition, an online training procedure to be administered to the caregivers of 22 patients participating in the OBSERV-AHC study using the illustrative and test cases was established. The illustrative cases were shown to caregivers and then the test cases were given. Wilcoxon test was used to compare pre-test and post-test results (number identified correctly of the three test video). Cohen’s Kappa was used for degree of agreement with the experts. Likert Scale, with confidence interval calculation, was used to assess caregivers’ opinion on how helpful the procedure was. Calendar Reliability Study: An event calendar was developed by a panel of caregivers and experts in the field and provided as a paper version to 13 caregivers of patients included in the OBSERV-AHC Study in the USA center with weekly video-conference meeting to go over the documented events. The percentage of entries not needing any corrections, completions or modifications was tracked over an 8 week follow up period. ANOVA was used to test for any changes in the percentages of completions over the 8-week period. The Event Calendar was also implemented online, to be used by the caregivers of the European centers, to record the events through their mobile phones or PCs. The usefulness of the electronic version is the subject of another study by the consortium and this abstract delineates the reliability of the paper version, with weekly follow-ups, of the calendar.
Results: Video Library Training results: 1) Wilcoxon test showed improvement in identification of spells by caregivers (N=22 caregivers from multiple centers, mean + SE 2.57+0.18 before and 2.96+0.44 after, p=0.047). 2) Cohen’s Kappa showed high degree of agreement with the experts’ classifications (0.91 after completion of the initial training and 1.0 after retraining, with >0.8 considered as excellent agreement). 3) Likert Scale analysis showed that caregivers indicated the training was helpful (confidence interval: 1.236-1.854 without overlap with 0, indicating a statistically significant perceived benefit.). Calendar Reliability Results: 1) With the weekly review (N=13 from the USA center) 5.52% of entries needed clarification and 1.87% of entries needed correction while only 3.58% of entries continued to have missing information. Thus, 89.03% did not need any modification and 97.07% of entries were complete after the review. 2) The high percentage of complete information within the calendar did not change over the 8 weeks of follow up (96.36%, 96.36%, 95.24%, 97.14%, 97.14%, 97.14%, 100% and 97.14 % for weeks 1 through 8 respectively, p=0.804).
Conclusions: Video library training is a useful and viable way to train caregivers to accurately identify AHC events, and the calendar methodology is a useful and reliable method for record keeping of spells.
Acknowledgements: We thank the following AHC parent associations for their support of the OBSERV-AHC Study, with this Event Calendar sub-project, and of the IAHCRC-CLOUD Platform, serving the data collection for the OBSERV-AHC Study, the Videolibrary and the Training Procedure and the online Event Calendar: USA CureAHC and AHC Foundation, French, Icelandic, Italian, Spanish, UK, German, Dutch and Polish AHC Foundations
- Characterisation of a novel ATP1a3D923Y mouse model of alternating hemiplegia of childhood8
Pariya Anongjanya1, Paul Armstrong1, Sylvain Gigout1, Rikke Holm2, Nikita Gamper1, Bente Vilsen2, Steven Clapcote1
Introduction: Alternating hemiplegia of childhood (AHC; OMIM 614820) is a rare neurological disorder characterised by transient episodes of hemiplegia, dystonia, abnormal eye movements, and seizures. De novo missense mutations in the ATP1A3 gene are the most common cause of AHC. Our current research focuses on the Asp923Tyr (D923Y) mutation identified in two AHC patients to date. Molecular modelling of the D923Y mutation has shown severe structural effects on Na,K-ATPase (NKA) α3, including impaired K+ movement along the K+ access pathway. We have developed an Atp1a3-D923Y mouse model in which we are assessing the phenotypic effects of this mutation.
Methods: A battery of behavioural tests was conducted on Atp1a3D923Y/+ (D923Y) and wild type (WT) littermates. The test battery consisted of the open field (OF), elevated plus maze (EPM), trace fear conditioning (TF), light dark box (LD) and Y-maze (YM) tests. Functional analysis of brain NKA activity was performed on snap frozen tissue samples following behavioural testing. Electrophysiological measurements in ex-vivo cortical slices from D923Y mice were also conducting.
Results: In the OF, D923Y mice spent significantly more time in the outer zone (p<0.01), suggesting less anxiety-like behaviour than WT mice. In the EPM, mice exhibited significantly more head dips on the open arms (p<0.001), suggestive of less anxiety. In TF, D923Y mice showed decreased freezing in response to the conditioned stimulus (auditory tone). No genotype differences were observed in the YM and LD tests. In NKA activity assays, D923Y mice showed significantly less (27.8% lower than WT) total brain NKA activity than WT mice (p<0.0001). Electrophysiological analysis in D923Y mice showed an increased amplitude of field potentials (p<0.001) and a shorter latency to peak of field potentials (p<0.05) in upper cortical layers following electrical stimulation of the lower cortical layers.
Discussion: The results highlight altered anxiety-related behaviour in the D923Y mice compared to WT controls. The apparently contradictory results in the OF and EPM tests could be due to a difference in mouse age between the two experiments groups (4.5-5 months vs 2 months). No differences in spatial working memory were found in the YM, however, hippocampal-dependent long-term memory was impaired in the TF test. Brain NKA activity was reduced, and alterations in cortical activity were observed in D923Y mice compared to WT controls.
Conclusion: The novel D923Y mouse model recapitulates some of the symptoms seen in the human disorder, however, further investigation is required to give a comprehensive assessment. This model is a valuable tool for future research into the mechanisms and treatment of AHC.
- E: Episode Tracking App for AHC9
Dr. Stephen A. Henderson
Introduction: Using Agile Development Techniques, five undergraduate students at William Penn University in the United States partnered with their adjunct professor, Mr. Rob Hammann, a Software Engineer Manager at Vermeer Manufacturing, and Dr. Stephen Henderson, father of Estella, who was diagnosed with Alternating Hemiplegia of Childhood, to develop E, an AHC episode tracking app. E, a parent-designed tool, aims to assist medical treatment and deliver healthcare providers quantitative data to inform and improve care for AHC patients.
Methods: The creation of E, the first AHC episode recording app, was completed using the agile software development method. Multiple methodologies exist within the agile framework. For this project the team utilized the scrum method, which moves projects forward by improving communication between team members and breaking down large quantities of work into smaller, more manageable tasks known as sprints. A sprint is an iterative development cycle, and in the case of E, it happened in two-week increments. At the end of each sprint, the team presented completed tasks in a demonstration to the product owner. The team worked in two-week sprints over 16 weeks using all these methodologies.
*Ethics was not required.
Results: The team is producing a functioning app that is currently still in the development phase. Current features include patient profiles, episode recording, medication management, exportable reports, and a help page.
Discussion: Future work consists of aggregating collected data, producing user-friendly reports, creating a mobile wrapper, data management across international borders, and authorizing a super-administrator to manage all symptoms.
Product Co-owners: Rob Hammann, Software Engineer Manager, Vermeer Manufacturing & Dr. Stephen A. Henderson, Associate Dean for Academic Affairs, Chair-Education Division, William Penn University, and AHCF Board Secretary.
Developers: Anibal Gonzalez, Kaung Htet-Thar, Jace Lukefahr, Chimezie Okeke, Carly Spikes
- A fever-induced neurological syndrome is caused by the temperature-sensitivity of an ATP1A3 mutation, p.Arg756His10
Elena Arystarkhova1, Mads S. Toustrup-Jensen2, Rikke Holm2, Jae-Kyun Ko3, Kyung Eun Lee3, Polina Feschenko1, Laurie J. Ozelius1, Allison Brashear4, Bente Vilsen2,and Kathleen J. Sweadner1
ATP1A3 is the gene encoding the α3 isoform of Na,K-ATPase. In the CNS it is expressed only in neurons. Human mutations produce a wide spectrum of phenotypes, but particular syndromes are associated with unique substitutions. For arginine 756, three different substitutions produce encephalopathies that manifest during febrile infections. Here we tested the pathogenicity of p.Arg756His (R756H) expressed in mammalian cells. R756H had sufficient transport activity to support cells when endogenous ATP1A1 was inhibited. It had approximately half of the turnover rate of WT with reduced affinity for Na+ and increased affinity for K+. There was evidence for moderate ER retention during biosynthesis at 37oC, indicating misfolding, and only modest benefit from the folding drug phenylbutyrate (4-PBA). When cells were incubated at 39oC, however, in the febrile range, α3 protein level dropped significantly without loss of the beta subunit, paralleled by an increase of endogenous α1. Elevated temperature resulted in internalization of α3 from the surface with some β subunit, accompanied by cytoplasmic redistribution of LAMP1, a marker of lysosomes and endosomes. After a return to 37oC, there was recovery of α3 protein levels, sensitive to cycloheximide. Heating in vitro showed mutation-specific activity loss at a rate 20- to 30-fold faster than the wild type, indicating a temperature-dependent destabilization of the protein structure. The observations are consistent with fever-induced variable symptoms in patients followed by recovery, with some accumulation of persistent symptoms. Arg756 appears to confer thermal resistance by forming hydrogen bonds among four separate parts of the complex Na,K-ATPase structure.
- Neuromodulation of sodium potassium ATPase pumps dynamically regulate mammalian spinal networks11
Struan Nisbet, Simon A Sharples and Gareth B Miles
The spinal cord contains motor networks that produce the rhythmic activities that underlie a range of locomotor behaviours. In order to produce these behaviours, spinal motor networks must be flexible to respond to changing requirements. Descending input from the brain can trigger these changes, often through neuromodulatory mechanisms, which can act via a variety of membrane targets, such as ion channels that govern intrinsic properties of the neurons within the locomotor network. Recently, a form of the sodium/potassium pump containing an isoform of the α subunit encoded by the ATP1A3 gene, has been suggested to dynamically alter neuronal output following periods of neuronal activity. At the cellular level, this leads to a period of inhibition following neuronal activity that lasts on the timescale of tens of seconds to a minute termed the usAHP. At the network level this mechanism may allow for dynamic inhibition of rhythmic activity and mounting evidence suggests that this mechanism can be adjusted by neuromodulation which may translate to altered network function. It is unknown currently the degree of contribution of the α3 pump and how this pump may be targeted by neuromodulation. In order to evaluate this, experiments were performed using isolated mouse spinal cords in which with rhythmic network activity can be monitored with extracellular neurograms obtained from ventral roots of the lumbar spinal cord. Rhythmic network activity was evoked by blocking fast inhibitory synaptic transmission, which was followed by subsequent application of the sodium ionophore monensin to increase intracellular sodium. This resulted in maximal activation of sodium/potassium pumps and, eventually, total inhibition of network activity. These experiments were then repeated in the presence of the neuromodulator dopamine, which has been previously demonstrated to increase usAHP activity. In the presence of dopamine, monensin-induced network inhibition occurred faster, reinforcing previous single cell findings at the network level. Additionally, these experiments were performed in a mutant mouse line that had a knockdown of functional ATP1A3 expression. In these mice the faciliatory effect of dopamine pump mediated inhibition was reduced. These results suggest that the α3 pump may be an important target for dopaminergic neuromodulation of network activity.
Poster Prizes
The Researchers and Clinicians from the Organising Committee will be assessing the poster submissions throughout the event. Prizes will be awarded on Friday 21st October.
ORGANISING COMMITTEE MEMBERS
We extend our thanks to the members of the organising committee.
Patient Organisation Representatives
Charlotte Audsley, AHC UK
Abhishek Behl, AHC UK, AHCFE
Katherine Behl, AHC UK, AHCFE
Jo Brown, AHC UK
Sigurður Jóhannesson, AHC Iceland, AHCFE
Sue Kemp, AHC UK
Nienke Lentze, AHC Netherlands
Josh Marszalek, AHC Foundation USA
Marek Parowicz, AHC 18+
Mirjana Pavlicek, AFHA
Vicky Platt, AHC Foundation USA
Ilona Skibinska-Mamzer, Polish Association, ahc-pl
Rosaria Vavassori, AHC 18+, epiCARE
Bridget Vranckx, AESHA, AHCFE
Katelyn Wilson, AHC Foundation USA
Researcher and Clinician Representatives
Dr Simona Balestrini
Dr Steve Clapcote
Professor Helen Cross
Professor Sanjay Sisodiya
Professor Arn Van den Maagdenberg
Dr Ailsa McLellan
Dr Aikaterini Vezyroglou
Our thanks also to those who have helped the conference run smoothly.
Conference Support including Michelle Koshy, Cameron Norton, Ellie, Deborah Audsley, Olivia Currie and Kristen Murray
Rapporteurs: Dr Alexander Simpson and Dr Matthew Pendleton
FUNDERS
We would like to offer our sincere thanks to those organisations who have been able to financially support this event.
European Joint Programme on Rare Diseases
Alternating AHC Iceland
AHC Foundation, USA
AHC Netherlands
AESHA
AFHA
AISEA
REMEMBERING OUR CHAMPIONS
This event is for all the AHC/ATP1A3 Champions; those with us and those who have died. On the last day of this event, we will remember them and their families.
You continue to inspire us with your smiles and laughter amongst all that you endure.
We commit ourselves, as families, researchers, and clinicians, to continue our work and our fight to improve your quality of life, and in the coming years, end these diseases. We will never give up.
“Parents learn a lot from their children about coping with life.”
Muriel Spark, Edinburgh author
The Comforters (1957)
REMEMBERING
A message from Dominique Poncelin
Former Président of French AHC Family Group AFHA
I would say that during these past 31 years of international links with people concerned by AHC, my best memories are:
The discovery of the gene ATP1A3 as main responsible for AHC which lead to three important consequences:
- AHC is a genetic rare disease with the hope to find a treatment in the future.
- Considering that mutations are de novo mutations :
- Parents having a child with AHC may keep in mind to have other children with a very very low probability to get a other child with AHC
- Siblings of an AHC child have no probability to get a child with AHC in the future
- I also learned that for such a rare disorder, a collaboration between scientists and family groups at an international level is essential and compulsory: the best example is gene discovery which has been possible thanks to a collaboration between an American research team who found the first mutation on 8 AHC cases and AHC family groups from USA and Europe who sent over 150 blood samples to confirm the discovery officially.
- However, I do not forget that AHC may become sometimes a devastating disease with a poor outcome and more than 50 of our soldiers fighting against AHC already passed away like my son Patrick after almost 35 years.
We must never forget them.
Doctors and scientists involved in AHC/ ATP1A3 research must speed up their collaboration and work , keeping in mind that scale of time is not the same for them and us :
1 year is almost nothing for research (12 years between the first blood bank and gene discovery for AHC)
But 1 year means 365 days of stressful or worrying situations for families due to the daily Life (attacks / seizures / behaviour troubles / emergency rooms and so on.)
Thank you to all of you for your interest and involvement in AHC research at different levels.
My best wishes with special thoughts to AHC kids or adults and those who already left us too early.
CALL FOR ORGANISING
THE 2023 ATP1A3 IN DISEASE SYMPOSIUM
Hendrik Rosewich, Karin Lykke-Hartmann, Kevin C. Ess and Tsveta Schyns-Liharska
The Standing Committee for the ATP1A3 in Disease Symposium
www.atp1a3-disease-symposium.org
The Standing Committee (SC) was formally established at the London meeting 24-26 August 2016 to ensure that the Mission and the Vision of the Symposium will be achieved. It is entirely voluntarily and informal structure of dedicated individuals from the ATP1A3 Community. It is a permanently acting group that is different from the Organizing Committees set up for each of the annual meetings. The tasks of the SC include: 1) To network, identify and select the hosts for the annual meetings; 2) To advise and assist the Organizing Committee on the Program and with the organization of the meeting in accordance with the Objectives of the Symposium; 3) To archive and make publicly available information on the past meetings; 4) To maintain a dedicated web site and emailing list of the Symposium; 5) To communicate the outcomes of the meetings and to promote and advertise the Mission and the Vision of the Symposium.
At the Edinburgh meeting 19-21 October 2022 the Standing Committee invites those who are interested in organizing and hosting the 11th Annual Symposium on ATP1A3 in Disease to freely contact the members of the SC or/and to send their expression of interest to atp1a3disease@gmail.com by latest 22 November 2022.



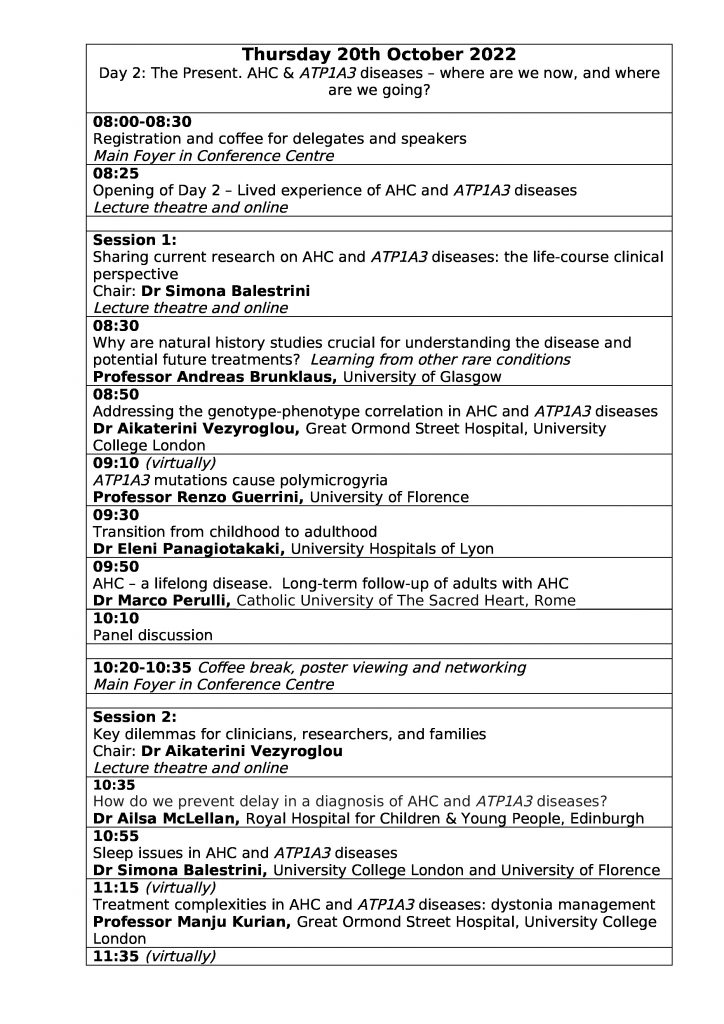


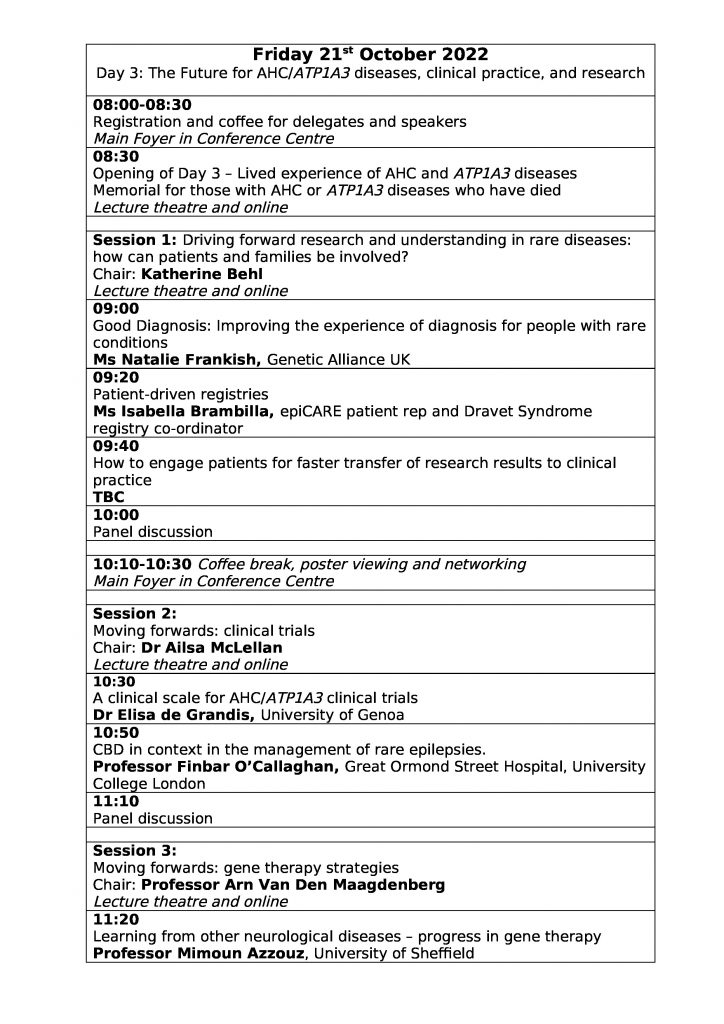
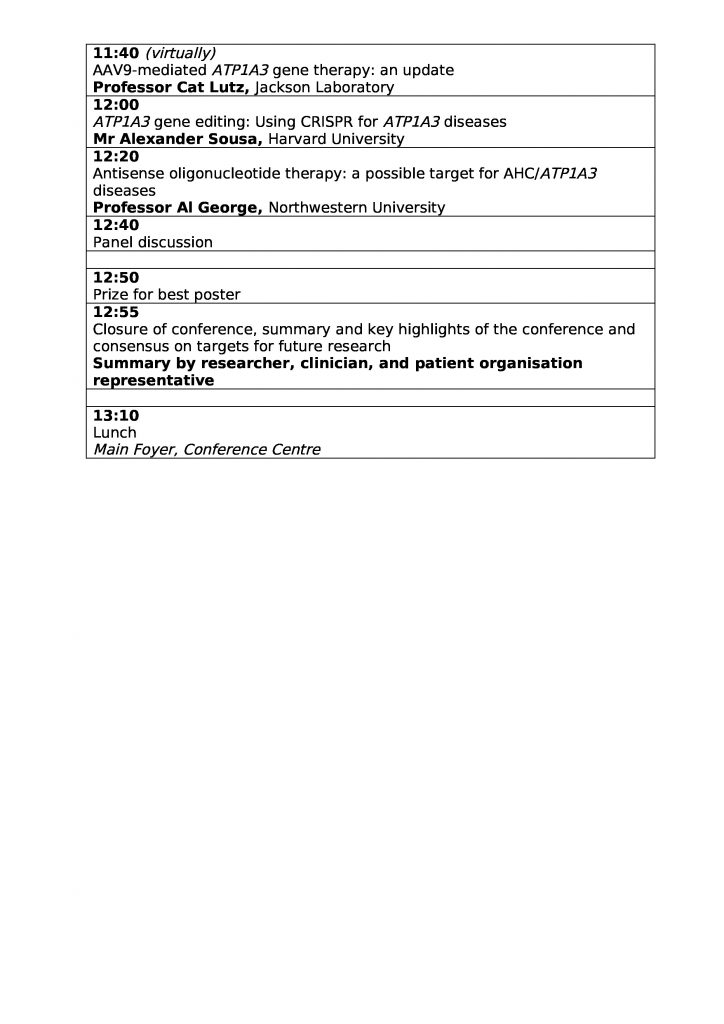

You must be logged in to post a comment.